Abstract
We established a national audit to assess the thromboprophylaxis rate for venous thromoembolism (VTE) in at risk medical patients in acute hospitals in the Republic of Ireland and to determine whether the use of stickers to alert physicians regarding thromboprophylaxis would double the rate prophylaxis in a follow-up audit. 651 acute medical admission patients in the first audit and 524 in the second re-audit were recruited. The mean age was 66.5yrs with similar numbers of male and female patients and 265(22.6%) patients were active smokers. The first and second audits identified 549(84%) and 487(93%) of patients at-risk for VTE respectively. Of the at-risk patients, 163(29.7%) and 132(27.1%) received LMWH in the first and second audit respectively. Mechanical thromboprophylaxis was instigated in 75(13.6%) patients in the first and 86(17.7%) patients in the second audit. The placement of stickers in patient charts didn’t produce a significant increase in the number of at risk patients treated in the second audit. There is unacceptably low adherence to the ACCP guidelines in Ireland and more complex intervention than chart reminders are required to improve compliance.
Introduction
Acute VTE occurs in medical inpatients, contributing significantly to morbidity and mortality1-4. In the UK, it’s estimated that up to 25,000 hospital death per year are due to pulmonary embolism (PE)5. Of the cases of fatal PE, 75% occur in hospitalized medical patients who weren’t receiving prophylaxis5. The frequency of confirmed DVT is 10-20% in hospitalized patients and can be significantly reduced through prophylaxis6. Cochrane7 and NICE8 have reviewed the importance of VTE-prophylaxis. LMWH treatment reduced the incidence of DVT by 60%, and symptomatic PE by 39% compared to placebo (RR 0.61, 95%CI 0.25,1.5)7,8. Hospital policies regarding thromboprophylaxis vary from mandatory intervention to physicians’ discretion. Some audits suggest that physicians have begun to recognize VTE as a serious health problem and use prophylaxis for high-risk patients8. Nevertheless, VTE prophylaxis remains underutilized in many centres9.
The objectives of this audit were to determine (1) the background rate of patients at-risk in representative hospitals throughout Ireland (2) the rate of VTE prophylaxis in those patients (3) if we would double the rate of VTE prophylaxis in at-risk medical patients by repeating the audit following the introduction of a specific physician reminder program using stickers in the charts of new medical admissions.
Methods
Acute medical patients, after written consent was obtained, were enrolled. Data were obtained from medical records. The same non-consultant hospital doctor (NCHD) completed both audits. Each audit had to be completed within five days of the first patient enrolment. The ACCP guidelines for thromboprophylaxis and the Cohen model for completion of VTE risk assessment7 were provided. Exclusion criteria included following patients: psychiatric, pediatric, palliative, maternity/obstetrics, neonatal, burn, ear nose and throat, dermatological, ophthalmologic, alcohol/drug treatment, rehabilitation patients and those admitted by the surgical teams. Patients admitted for treatment of DVT or PE (begun <24 hours of admission) or admitted for diagnostic testing only were excluded.
The two audits were separated by 3 months. During the five days of each audit a pre-printed sticker was placed in the chart informing the team that the patient was participating in an audit, and recorded if VTE assessment was completed. A second pre-printed sticker was inserted in the local prescription card with a direction on therapeutic intervention. A third sticker was used to remind relevant medical personnel that the patient participated in the study and that follow-up and treatment may be warranted. Following the first audit, specific stickers were placed in the charts of all new admissions over a three-month period, raising awareness and increasing the rate of medical prophylaxis. It was assumed for the power calculations that approximately 39% of patients included in the study would be at risk of VTE6. It was recommended that at least fifty patients be recruited in both audit periods in each centre, allowing detection of a doubling of the intervention rate (medical prophylaxis) from a 10% to 20% with power of 90%.
The rates of risk assessment and VTE intervention were summarized for each centre and overall across all hospitals for each study period. For the rate of risk assessment, the planned analysis was a proportional odds model to estimate the odds ratio of risk assessment being done versus recommended/not done for the second audit relative to the first. An overall estimate of the rate of risk assessment performed was also derived using a mixed model analysis treating the centers as random effects. The change in the rate of intervention from the first to the second audit was analyzed using a logistic regression model fitted with terms for centre and audit period to see if centers differed for the change in intervention rate. The data show the odds ratio of final intervention rate to initial intervention rate, with 95% confidence intervals. This indicates whether the change seen was similar across all participating centers, or whether there were some centers performing differently to the others. All statistical analyses were carried out using SAS Version 9.2 for Windows.
Results
Study Population
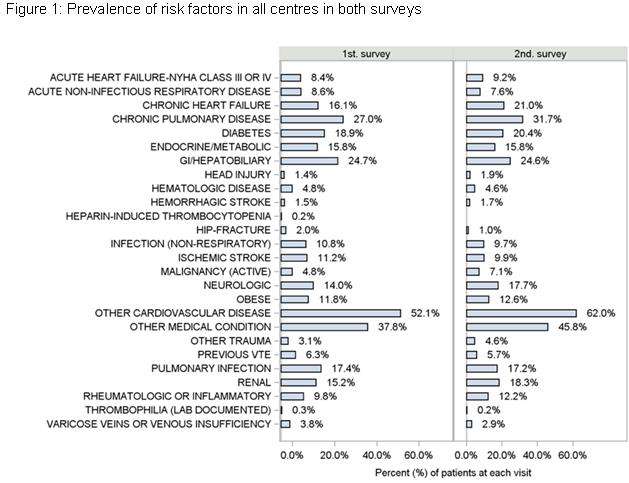
11 acute hospitals participated, recruiting 651 patients during the first audit and 524 in the second; one centre did not perform the second audit and as a result was excluded from analyses. The number of patients recruited by each centre ranged from 38 to 98 in the first audit and from 22 to 100 in the second. Six patients were excluded from all analyses; three did not give informed consent and three didn’t meet the study inclusion criteria. In the first audit period, the mean age of patients was 65.6(±18.1)yrs. In the second audit the mean age was 67.5(±16.3)yrs. In both audits, there were equal numbers of males and female patients. The age and sex distribution of the patients studied in the two audits were similar across all centers. In the first and second audits 21.8% and 23.5% of patients respectively were actively smoking. Smoking status was also similar for the two audits. The prevalence of all chronic diseases documented was similar within centers between the two audits (Figure 1).
Risk Assessment in Patients At Risk of VTE
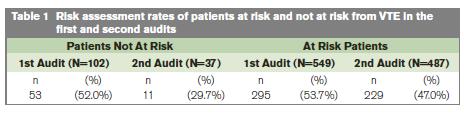
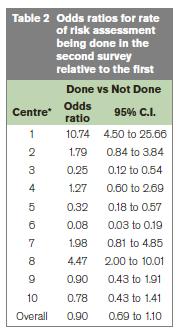
In the first and second audits 84% and 93% of patients were considered at risk of VTE respectively. The rate of risk assessment being completed and documented in the medical record for those patients considered at risk of VTE was 53.7% and 47.0% in the first and second audits, respectively (Table 1). With the exception of 2 sites, the results for each centre are all consistent with the overall estimate. The overall estimate of the probability of risk assessment being done at the first visit was 0.53 and 0.50 at the second visit, giving an odds ratio for the difference between visits of 0.90 (95% C.I. 0.69 to 1.10) (Table 2).
VTE intervention
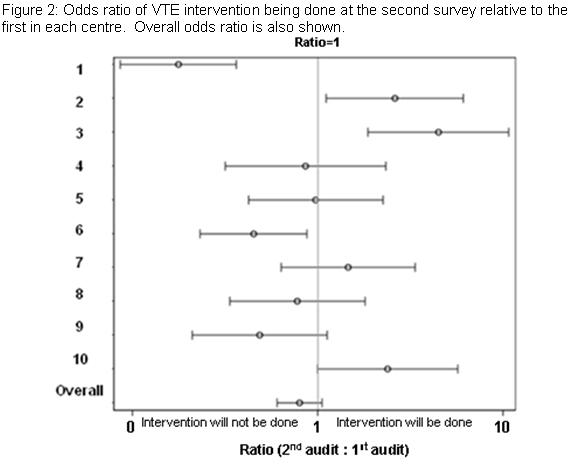
The patients who were at risk of VTE were significantly more likely to have VTE intervention than those who were not (p<0.0001); the odds of having intervention for the at-risk patients being around three times higher than those not at risk. There was a statistically significant interaction between centre and the audits (p<0.0001) in terms of the rate of intervention. The rates of intervention increased in the second survey for three of the hospitals. The rates for three of the hospitals declined. The rates in the other centers were similar for the two audits. Fitting the centers as random in order to derive an overall estimate for the at-risk patients of the rate of intervention during the second audit relative to the first yielded no statistically significant difference (p=0.11). The odds ratio for the odds of intervention at the second visit relative to the first was 0.80 (95% C.I. 0.60 to 1.05) (Figure 2). The number of at-risk patients receiving LMWH was 27.9% for first audit and 27.1% for the second. The number of at-risk patients receiving mechanical prophylaxis increased from 13.6% in the first audit to 17.7% in the second audit.
Discussion
This national study showed that the use of VTE-prophylaxis was sub-optimal in Ireland. While at-risk patients were more likely to have a VTE intervention, only 27% of this at-risk group was actually commenced on LMWH. The uptake in the participating hospitals of ACCP recommendations was sub-optimal. Attachment of stickers to drug charts was not sufficient to ensure commencement of VTE-prophylaxis. The overall number of patients considered at risk of developing VTE (close to 90%) was higher than anticipated. The study was planned to detect a doubling of the intervention rate, based on an initial intervention rate of between 10% and 40%. In this study the lowest rate of intervention during the first survey period was 34% only in one centre whereas in remaining centers the rates were >50%. It is well recognized that acutely ill patients have an increased risk of developing VTE10. The ENDORSE Global study has reported a range of 21.1% to 71.2%11. In our study 84% and 94% of our patients were in the at risk category. This could be accountable by an older population with multiple co-morbidities presenting in our hospitals giving these patients a higher risk profile.
The inconsistent use of prophylactic measures for VTE in hospital patients seen in our study has been widely reported. A UK survey suggested that 71% of patients assessed to be at medium or high risk of developing deep vein thrombosis did not receive any form of mechanical or pharmacological VTE prophylaxis3. The French experience showed that of the 50% of at risk patients, 38.6% did not receive thromobo-prophylaxis12. Globally, the findings of the International ENDORSE study indicated that up to 50% of all hospitalized patients at-risk for VTE were not receiving appropriate prophylaxis13. Furthermore, medical patients at-risk for VTE were less likely than surgical patients to receive appropriate prophylaxis. A larger study spanning 32 countries showed 70% of the hospitalized medical patients, < 50 years of age did not receive VTE prophylaxis11. Between audits there was no increase in the use of VTE-prophylaxis despite the increased prevalence risk factors including cardiovascular disease and chronic pulmonary disease. Interestingly many patients not at risk received thromboprophylaxis treatments (data not shown). Implementation of VTE-prophylaxis may be confounded by several factors including confusion regarding the assessment of VTE-risk, lack of awareness of VTE-risk and complexity associated with the patients.
Other authors have shown improvement in VTE prophylaxis rates using similar sticker-based approaches14,15. In contrast to our study, these studies used a single site hospital; or survey encompassed 11 hospital sites throughout Ireland. The New-Zealand based survey utilised pharmacist to identify patients who were suitable for thromboprophylaxis, prompting physicians to consider LMWH and recommending doses after performing risk assessments14. Three trials have guided the use of VTE-prophylaxis in hospitalized patients: MEDENOX16, PREVENT17 and ARTEMIS18. The population cohort of these trials may have been biased in selecting patients who were at high risk and who had a low bleeding risk. Our doctors in contrast may be dealing with elderly frail patients with high risk of falls and bleeding. Hence there may some reluctance in prescribing the LMWH.
The recommendations’ from the ACCP for thromboprophylaxis apply to patients with congestive cardiac failure or severe respiratory disease confined to bed and have one or more additional venous thromboembolism risk factors and those admitted to ICU6. Other risk stratification systems including those described NICE19 and other authors20-24 are available however none of these have been validated in clinical trials. The implementation of a standardized national protocol in Ireland for risk assessment and prophylaxis prevention of VTE is lacking even though it remains an ACCP Grade 1A recommendation. In the UK, financial incentives for organizations have been introduced for Quality and Innovative Payment Framework (CQUINN). A proportion of CQUINN payments to acute providers are conditional on risk assessing at least 90% of patients admitted to hospital25. VTE-prophylaxis champions, be it nursing, pharmacists or physicians may be required at on each ward to improve rates. Urgent national support and prioritization of VTE-prevention led by clinicians and multidisciplinary teams are essential to ensure that the protocols are in place.
Correspondence: S Gaine
Mater Misericordiae University Hospital, Eccles St, Dublin 7
Email: [email protected]
Acknowledgements
PREVENT VTE Investigators: M Tariq2, S Foley3, D O'Keeffe4, P Murphy5, J Doherty6, J Connaughton7, E Mulloy8, J Faul9, R Costello10, V Keatings11, MT O’Connell 1. This study was supported by an unrestricted research grant provided by sanofi-aventis Ireland Ltd. The technical and statistical support of P O’Grady (Sanofi) is gratefully acknowledged.
References
1. Lindblad B, Sternby NH, and Bergqvist D. Incidence of venous thromboembolism verified by necroscopy over 30 years. BMJ 1991; 302:709-711.
2. Sandler DA, Martin JF. Autopsy proven pulmonary embolism in hospital patients: are we detecting enough deep vein thrombosis? J R Soc Med 1989; 82:203-205.
3. Rashid ST, Thursz MR, Razvi NA, Voller R, Orchard T, Rashid ST, Shlebak AA. Venous thromboprophylaxis in UK medical inpatients. J R Soc Med 2005;11:507–12.
4. Cohen AT, Edmondson RA, Phillips MJ, Ward VP, and Kakkar VV. The changing pattern of venous thromboembolic disease. Haemostasis 1996; 26: 65-71.
5. House of Commons Health Committee. The prevention of venous thromboembolism in hospitalised patients. 2005. London, Department of Health
6. Geerts WH, Bergqvist D, Pineo GF, Heit JA, Samama CM, Lassen MR, Colwell CW; American College of Chest Physicians. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008;133:381S-453S.
7. Alikhan R, Cohen AT. Heparin for the prevention of venous thromboembolism in general medical patients. Cochrane Database Syst Rev 2009 Issue 3.
8. Amin AN, Stemkowski S, Lin J, Yang G. Preventing venous thromboembolism in US hospitals: Are surgical patients receiving appropriate prophylaxis? Thromb Haemost 2008; 99:796-797.
9. Yu H.T, Dylan ML, Lin J, Dubois RW. Hospitals' compliance with prophylaxis guidelines for venous thromboembolism. Am J Health Syst Pharm 2007; 64: 69-76.
10. Cohen AT, Alikhan R, Arcelus JI, Bergmann JF, Haas S, Merli GJ, Spyropoulos AC, Tapson VF, Turpie AG. Assessment of venous thromboembolism risk and the benefits of thromboprophylaxis in medical patients. Thromb Haemost 2005; 94:750-759.
11. Anderson FA Jr, Goldhaber SZ, Tapson VF, Bergmann JF, Kakkar AK, Deslandes B, Huang W, Cohen AT. ENDORSE Investigators. Venous thromboembolism risk and prophylaxis in hospitalised medically ill patients. The ENDORSE Global Survey. Thromb Haemost 2010;103:736-48.
12. Bergmann JF, Lloret-Linares C, Rami A, Cohen AT, Garay RP, Kakkar AK, Goldhaber SZ, Deslandes B, Tapson VF, Anderson FA; pour les investigateurs de l’étude Endorse France. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): results obtained in France]. Presse Med 2011;40:e528-37.
13. Cohen AT, Tapson VF, Bergmann JF, Goldhaber SZ, Kakkar AK, Deslandes B, Huang W, Zayaruzny M, Emery L, Anderson FA Jr; ENDORSE Investigators. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross-sectional study. Lancet 2008;371:387-94.
14. Gladding P, Larsen F, Durrant H, Black P. Education together with a preprinted sticker improves the prescribing of prophylactic enoxaparin. NZMJ 2007;120:U2461..
15. Sharif-Kashani B, Raeissi S, Bikdeli B, Shahabi P, Behzadnia N, Saliminejad L, Samiei-Nejad M, Nasiri F, Khayyami M, Forootan B, Pozhan S, Masjedi MR. Sticker reminders improve thromboprophylaxis appropriateness in hospitalized patients. Thromb Res 2010;126:211-6.
16. Samama Samama MM, Cohen AT, Darmon JY, Desjardins L, Eldor A, Janbon C, Leizorovicz A, Nguyen H, Olsson CG, Turpie AG, Weisslinger N. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group. N Engl J Med 1999;341:793–800.
17. Leizorovicz A, Cohen AT, Turpie AG, Olsson CG, Vaitkus PT, Goldhaber SZ; PREVENT Medical Thromboprophylaxis Study Group. Randomized, placebo-controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients. Circulation 2004;110:874–9.
18. Cohen AT, Davidson BL, Gallus AS, Lassen MR, Prins MH, Tomkowski W, Turpie AG, Egberts JF, Lensing AW; ARTEMIS Investigators. Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trial. BMJ 2006;332:325–9.
19. Venous thromboembolism: reducing the risk of venous thromboembolism in patients admitted to hospital. National Clinical Guideline Centre Jan 2010. http://guidance.nice.org.uk/CG92.
20. Kucher N, Koo S, Quiroz R, Cooper JM, Paterno MD, Soukonnikov B, Goldhaber SZ. Electronic alerts to prevent venous thromboembolism among hospitalized patients. N Engl J Med 2005;352:969-977.
21. Lecumberri R, Marqués M, Díaz-Navarlaz MT, Panizo E, Toledo J, García-Mouriz A, Páramo JA. Maintained effectiveness of an electronic alert system to prevent venous thromboembolism among hospitalized patients. Thromb Haemost 2008;100:699–704.
22. Barbar S, Noventa F, Rossetto V, Ferrari A, Brandolin B, Perlati M, De Bon E, Tormene D, Pagnan A, Prandoni P. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the Padua Prediction Score. J Thromb Haemost 2010;8:2450–2457.
23. Rocha AT, Paiva EF, Lichtenstein A, Milani J, Cavalheiro-Filho C, Maffei FH. Risk-assessment algorithm and recommendations for venous thromboembolism prophylaxis in medical patients. Vasc Health Risk Manag 2007;3:533–553.
24. Bullock-Palmer R. P., Weiss S., Hyman C. Innovative approaches to increase deep vein thrombosis prophylaxis rate resulting in a decrease in hospital- acquired deep vein thrombosis at a tertiary-care teaching hospital. J Hosp Med 2008;3:148-155.
25. Venous Thromboembolism Prevention. A Guide for Delivering the CQUIN Goal http://www.thrombosischarity.org.uk/cms/images/stories/File/Guides/Guide%20for%20Delivering%20CQUIN%20-%20Small%20PDF.pdf