Ir Med J. 2007 Nov-Dec;100(10):631-2
M Falconer, D Molloy, J Ingerhaug, M Barry
St James’s Hospital, Dublin 8
Abstract
Adverse drug reactions account for approximately 5% of acute medical admissions. A 34-year-old male patient receiving antiretroviral therapy, methadone and flurazepam presented to the Emergency Room following collapse with associated loss of consciousness. Cardiac monitoring demonstrated marked Q-T prolongation followed by the cardiac arrhythmia, torsade de pointes. The patient made a full recovery following withdrawal of the antiretroviral therapy and a reduction in methadone dose. Methadone is a recognised cause of this potentially fatal cardiac arrhythmia which is more likely to occur when methadone metabolism is inhibited by drugs such as HIV protease inhibitors.
Introduction
Prolongation of the Q-T interval associated with polymorphic ventricular tachycardia, or torsade de pointes can be fatal. The pause dependence as seen in Figure 1; panel a, is typical of drug related torsade de pointes. Prolongation of the Q-T interval beyond 500 ms is regarded as conferring increased risk. Drugs that are commonly associated with this arrhythmia include disopyramide, procainamide, quinidine, sotalol and amiodarone. Antiinfective agents (clarithromycin, erythromycin), antiemetics (domperidone, droperidol), antipsychotics (chlorpromazine, haloperidol) and methadone, particularly in high concentrations are also associated1.
Case Report
In September 2007 a 34-year-old male patient was admitted to the Emergency Room having collapsed with associated loss of consciousness following ingestion of ten flurazepam 15mg tablets. Clinical examination revealed drowsiness, meiosis, sweating and right basal inspiratory crepitations. He was afebrile with a respiratory rate of 22 per minute and an oxygen saturation of 98%. The cardiograph demonstrated sinus rhythm at 68 beats per minute. Laboratory investigations including full blood count and biochemistry profile were normal save for a raised AST 54 IU/L and bilirubin 34 Ìmol/L. The patient had a history of intravenous drug use and was currently taking methadone 105 mg per day. He also had a history of hepatitis C and HIV disease and commenced antiretroviral therapy approximately 9 months prior to admission. He was unsure of the individual anti-HIV medications and admitted to poor compliance with these and prophylactic co-trimoxazole. He described a previous episode of collapse at home 3 weeks prior to admission and attributed this to concomitant ingestion of his antiretroviral medications plus flurazepam.
Antibiotic therapy was commenced with co-amoxiclav 625 mg twice daily in addition to intravenous fluids and oxygen. Three hours following presentation the patient developed sinus bradycardia with a ventricular rate of 39 beats per minute and a blood pressure of 90/50 mmHg. Whilst in the Emergency Room he received intravenous atropine 0.25 mg on two occasions resulting in an increase in heart rate to 56 beats per minute and a blood pressure of 103/67 mmHg. The patient was transferred to the medical ward and placed on telemetry. Eight hours following his initial presentation (2300 hrs) the patient was noted to have asymptomatic bradycardia with a ventricular rate of 35 beats per minute. The ECG showed a PR interval of 142 ms and a QT of 728 ms (QTc = 555 ms). Shortly afterwards a 4 beat run of polymorphic ventricular tachycardia, a pause followed by 3 sinus beats interrupted by another episode of polymorphic ventricular tachycardia (torsade de pointes) was observed (Figure 1 ; panel a). The patient was asymptomatic but 80 minutes later had a second episode of polymorphic ventricular tachycardia (Figure 1; panel b). Intravenous magnesium was administered and the ventricular rate remained between 30 to 40 beats per minute with the systolic blood pressure between 94 and 105 mmHg overnight. There were no further arrhythmias.
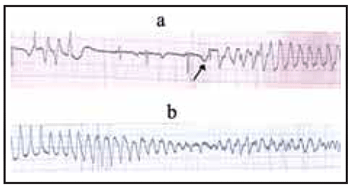 |
Figure 1 Methadone induced torsade de pointes:
Panel (a) shows a typical episode of drug induced
torsade de pointes with a four beat run of polymorphic
ventricular tachycardia, a pause, and a sinus beat
with along deformed QT interval (arrow) followed by
polymorphic ventricular tachycardia. Panel (b) shows
the ventricular tachycardia with beat to beat change
in the QRS axis often referred to as “twisting of the
points” hence the name - torsade de pointes. |
On the post take ward round we became aware of his antiretroviral therapy which included the protease inhibitors atazanavir 300 mg/day plus ritonavir 100 mg/day in addition to the combination of nucleoside reverse transcriptase inhibitors emtricitabine 200 mg + tenofovir 245 mg once daily (truvada 1 tablet daily). We discontinued the antiretroviral therapy because of the potential for interaction with methadone. Within 24 hours the QT interval reduced to 520 ms and after 48 hrs was 480 ms (QT was 504 ms on the first ECG). Echocardiography did not demonstrate any structural abnormality and an exercise ECG prior to discharge was normal. The patient preferred to stay on methadone and was discharged on a slightly lower dose of 90 mg/day. He was referred to the infectious diseases clinic for consideration of a new antiretroviral regimen.
Discussion
Methadone is extensively metabolised to the N – demethylated metabolite by the hepatic cytochrome P450 enzyme system. While cytochrome P450 2C8, 2C18 and 2D6 isoenzymes have a role in the demethylation of methadone cytochrome P450 3A4 is the major enzyme involved2. The HIV protease inhibitors atazanavir and particularly ritonavir are potent inhibitors of cytochrome P450 3A4 and coadministration would be expected to result in much higher concentrations of methadone with associated toxicity3. Flurazepam is likely metabolised by the same enzyme system thereby exacerbating the interaction. Truvada would not be expected to influence the metabolism of methadone. Withdrawal of the protease inhibitor reversed the QT prolongation in our patient. For those of us on acute medical call this case highlights the need to be aware of the association between methadone and the potentially fatal arrhythmia, torsade de pointes and for those prescribing antiretroviral therapy to be cognizant of the many drug-drug interactions involving the HIV protease inhibitors.
Correspondence:
M Barry
Department of Clinical Pharmacology & Therapeutics,
Trinity Centre for Health Sciences,
St James’s Hospital, Dublin 8
Email: [email protected]
References
- Roden D.Drug-induced prolongation of the QT interval N Engl J Med 2004;350:1013-1022
- Iribane C, Berthou F, Baird S, et al. Involvement of cytochrome P450 3A4 in the N-demethylation of methadone in human liver microsomes. Chem Res Toxicol 1996;9:365-373
- Barry M, Mulcahy F, Merry C, Gibbons S, Back D. Pharmacokinetics and potential interactions amongst antiretroviral agents used to treat HIV infection, Clin Pharmacokinet 1999;36:289-304