G Le1, P Lynam1, E Lawlor2, A O'Meara1, O Smith1, A O’Marcaigh1
1Department of Haematology, Our Lady’s Children’s Hospital, Crumlin, Dublin 12
2Irish Unrelated Bone Marrow Registry, National Blood Centre, Dublin 8
Abstract
Umbilical cord blood is being used increasingly as a source of haematopoietic stem cells for transplantation because of rapid availability, and the unavailability of a HLA matched adult donor for some patients. This study reports the characteristics and outcomes of 15 patients who have undergone umbilical cord blood transplantation (UCBT) in Ireland between 1998 and 2009. The median total nucleated cell and CD34+ doses post-processing were 6.5 x 107cells/kg and 1.8 x 105 cells/kg, respectively. Median neutrophil recovery time was 30 days (range, 14-44). Median platelet recovery time was 46.5 days (range, 35-148). 33.3% of patients developed acute cutaneous graft-versus-host disease (GVHD) grade I-II. Three patients died of transplant-related toxicity and two died of leukaemic relapse. We conclude that, with a satisfactory stem cell dose, UCBT offers a high chance of engraftment with acceptable toxicity, and should be regarded as a favourable option in selected patients when satisfactory bone marrow or peripheral blood stem cell donors are not available.
Introduction
Allogeneic transplantation using haematopoietic stem cells (HSC) from bone marrow or peripheral blood can be limited by the availability of HLA-matched related donors. Although 58% of Irish patients will have a matched related donor, only about 42 % of children under the age of 14 who require stem cell transplant will have such donors1. The majority of the remaining patients requiring transplant must receive transplant from an unrelated donor or a HLA-mismatched related donor. These patients are at a higher risk of developing acute and chronic graft-versus-host disease (GVHD)2. In recent years, the use of umbilical cord blood transplant (UCBT) has provided an alternative source of HSC for transplantation in a range of immunological, metabolic and haematological disorders. In 1988, the first UCBT was successfully performed in a child with Fanconi’s anemia. The cord blood belonged to the patient’s HLA-matched sister3. This success demonstrated that a single cord blood unit could contain enough HSC for haematopoietic reconstitution. Furthermore, it also showed that a cord blood unit could be collected at birth, cryopreserved, thawed at a later date, and then transplanted into a myeloablated host without causing the HSC to lose their regenerative potential3,4. The advantages of UCBT include ease of collection, rapid availability, a wider degree of permissive disparity between donors and recipients, and lower incidence of GVHD4,5. However, the major limitation of UCBT is that the limited number HSCs in a cord blood unit may result in delayed engraftment, thus precluding transplantation into larger recipients.
Many factors influence the likelihood of successful engraftment in UCBT, including total nucleated cell dose (TNC), nucleated cell viability, and colony-forming unit (CFU) activity6. A minimum TNC of 3.0 x 107 cells/kg is associated with higher engraftment rate4. Also, a threshold CD34+ cell count of at least 1.7 x 105 cells/kg correlates with a high probability of survival6. Recent studies supported using not only HLA-matched cord blood, but also one or two antigen HLA-mismatch cord blood in children needing transplant7,8. This report describes the clinical characteristics and outcomes of 15 patients with haematologic and metabolic disorders who have undergone UCBT in Ireland between 1998 and 2009.
Methods
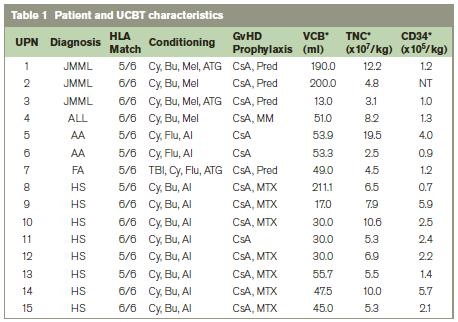
This study reviewed 15 patients who received UCBT from March 1998 to June 2009. Any patients who did not have fully HLA matched and CMV-matched bone marrow donors or peripheral blood stem cells (PBSC) donors were considered for UCBT. The underlying conditions included juvenile myelomonocytic leukaemia (JMML), relapsed acute lymphoblastic leukaemia,(ALL) aplastic anaemia (AA), Fanconi’s anaemia (FA) and Hurler syndrome (Mucopolysaccharidosis Type 1) (HS). 12 transplants occurred within one year of diagnosis. Informed consent was obtained from the legal guardians of all patients.Unrelated cord blood units (CBU) were sourced by the Irish Unrelated Bone Marrow Registry (IUBMR) at the Irish Blood Transfusion Service (IBTS) from international unrelated cord banks. Sibling cords were collected and stored in the IBTS Directed Cord Bank.
All CBU selected contained at least 2.5 x 107 total nucleated cells per kg recipient weight. HLA matching criteria for selection were a minimum of donor / patient matching at 4/6 loci by molecular typing methods based on HLA A and B typing at the antigen level, and DRB1 matched at the allelic level. Maternal and/or cord samples were tested for mandatory transfusion transmitted viral diseases and for cytomegalovirus (CMV). Conditioning regimens varied according to each patient’s disease. JMML patients received cyclophosphamide, busulphan, melphalan, and anti-thymocyte globulin (ATG). ALL patients received cyclophosphamide, busulphan, and melphalan. AA patients were given cyclophosphamide, fludarabine, and alemtuzumab. Patients with FA were treated with total body irradiation (TBI), cyclophosphamide, fludarabine, and ATG. Patients with HS were given cyclophosphamide, busulphan, and alemtuzumab (Table 1).
For GVHD prophylaxis, patients either received ciclosporin A alone or in combination with prednisolone, mycophenolate, or methotrexate. Patients were evaluated daily for acute GVHD while inpatient, and at outpatient clinics during the first 100 days post transplant. Diagnosis was based on clinical signs and histopathological evidence. Acute and chronic GVHD was graded according to the established criteria9. For supportive care, all patients were given prophylactic ursodeoxycholic acid (for veno-occlusive disease prevention), co-trimoxazole, aciclovir, and either voriconazole or liposomal amphotericin. Empirical broad spectrum antibiotics were administered as required. Each cord blood unit was processed (thawed and washed) according to the standard procedure from the New York National Cord Blood Program10, and then infused to the recipient through a central venous catheter. TNC, CD34+ cell count, and viability were re-assessed post processing prior to infusion. Donor/recipient ABO incompatibility was not a major concern because the majority of red blood cell and isohemagglutinins were removed in the washing process8,11.
Full blood counts were performed daily checking for haematopoietic recovery. Neutrophil recovery was defined as the first of 3 consecutive days with an absolute neutrophil count of > 0.5 x 109/L. Platelet engraftment time was defined as the first of 7 consecutive days with a platelet count of > 50 x 109/L without transfusion. After neutrophil recovery was achieved, engraftment status was determined by molecular chimerism assessment methods. The probability of 2-year-overall survival was estimated from the time of transplant using the Kaplan-Meier method.
Results
Three patients had JMML(UPN 1-3); one had relapsed infant ALL (UPN 4); two had AA (UPN 5, 6); one had FA (UPN 7); and eight had HS (UPN 8-15). The median age at transplant was 1.1 year (range, 0.3 – 10.0 years), and the median weight was 12.6 kg (range, 5.5-30.0 kg). One patient was seropositive for CMV and received a CMV positive donor cord blood unit from a CMV positive mother. Fourteen units were from unrelated donors, and one unit was from a sibling donor (UPN 2). Twenty percent of the donor/recipient pairs were ABO-matched. Eight units were fully matched (6/6) and seven units had one HLA antigen mismatch (5/6). The median TNC and CD34+ doses post-processing were 6.5 x 107cells/kg (range, 2.5-19.5) and 1.8 x 105 cells/kg (range, 0.7-5.9), respectively. Ninety-three percent of patients received a TNC dose >3.0 x 107 cells/kg.
The median time to neutrophil engraftment was 30 days (range, 14-44). Eighty-six percent of patients achieved neutrophil engraftment within 60 days post UCBT (Table 2). Two patients died before achieving neutrophil recovery. Sixty percent of patients achieved platelet engraftment by day 60. One patient reached platelet recovery at day 73, and another eventually achieved recovery at day 148. Patient UPN 7 had not reached platelet recovery at day 168 when this report was written. Median time to platelet recovery was 48 days (range 35-148). Molecular engraftment studies after neutrophil recovery showed donor engraftment ranging from eighty-seven to one hundred percent.
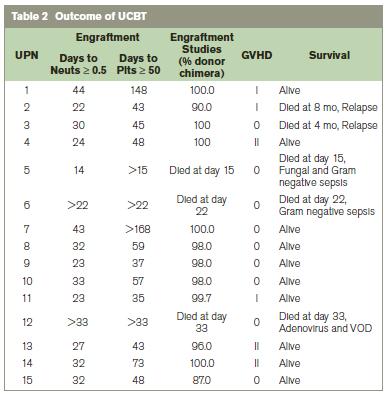
UPN: unique patient number; Neuts: neutrophils; Plts: platelets; VOD: veno-occlusive disease
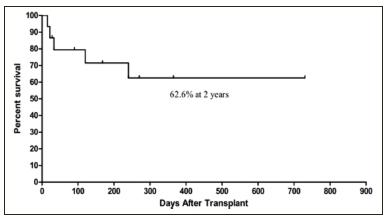
Two patients (UPN 5,6) died before neutrophil engraftment, and were not evaluable for GVHD. Of the 13 remaining evaluable patients, 6 (46%) had acute cutaneous GVHD grade I-II (Table 3). Only patients with grade II GVHD were treated with prednisolone, all successfully. No patients had grade III-IV GVHD or hepatic or intestinal GVHD. Two patients with JMML (UPN 2 and UPN 3) experienced relapse and died at 4 and 8 months. Two AA patients (UPN 5 and UPN 6) died of Gram negative sepsis at day 15 and day 22: one achieved only neutrophil engraftment at day 14, and the other had no evidence of haematopoietic recovery. One HS patient (UPN 12) died of adenovirus and veno-occlusive disease at day 33, also without haematopoietic recovery. Median follow-up time was 4.5 years. The overall survival was 62.6% at 2 years (Figure 1).
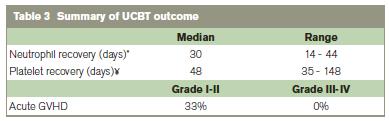
* Patient 6 and 12 did not reach neutrophil recovery and were not included
¥ Patient 5, 6, 7, 12 did not reach platelet recovery and were not included
Figure 1: Overall survival after UCBT
Discussion
We describe the results of single cord UCBT in 15 consecutive patients in a single paediatric centre. This review was undertaken to examine the clinical characteristics and outcome of UCBT, and to show its potential usefulness as an alternative source of stem cells when suitable bone marrow or PBSC are not available. Even though our UCBT patient population was small, it encompassed a wide range of conditions both benign and malignant. Thus, we were able to confirm that UCBT can be used in many haematological disorders and metabolic disorders.
We report the median neutrophil and platelet recovery times of 30 days and 48 days, respectively. These results are comparable with previous studies7,12. Time to haematological engraftment after UCBT is known to be longer than that after bone marrow transplantation5,7. Most of our patients achieved greater than ninety-five percent donor engraftment. Given the number of patients in our study, we did not observe a significant correlation between the TNC or the CD34+ doses and engraftment time. Other larger studies have documented that higher TNC and CD34+ doses improve neutrophil and platelet engraftment times4,6,8. This lack of correlation in our study could be attributed to the low number of patients in the cohort, or could suggest that the number of progenitor cells in the cord units exceed the threshold needed for engraftment in a paediatric patient14. As such, 93% of our patients received a TNC dose of greater than 3.0 x 107 cells/kg, which is the recommended level needed for a successful engraftment4. In this patient population, the overall incidence of acute GVHD was 46%. These patients developed only grade I-II cutaneous GVHD. Grade III-IV GVHD was not observed. Our experience is consistent with other studies that have demonstrated lower incidence of GVHD in umbilical cord blood recipients compared to bone marrow recipients5,13,14. The overall survival in this cohort at 2 year was 62.6%. This is in keeping with previous studies7,8. Seven patients out of 8 with HS survived and their long-term outcome is being monitored closely. Our survival curve shows that most deaths occurred within 250 days post UCBT. The causes of death were relapse (13%), infection (13%) and veno-occlusive disease (7%).
In summary, we have demonstrated that cryopreserved umbilical cord blood is a safe alternative source of hematopoietic stem cells that can be used for transplantation in paediatric patients. The high rate of engraftment and low incidence of grade III-IV acute GVHD despite HLA disparity make UCBT a favourable option when HLA-matched bone marrow or PBSC is not available. However, the major disadvantage of cord blood is the limited number of TNC compared to that of bone marrow. This drawback limits the use of UCBT in the adult population or in larger paediatric patients. To overcome this limitation, double cord transplantation and ex vivo stem cell expansion are currently in development15 to allow an expanded role for UCBT in both paediatric and adult patients.
Correspondence: A O'Marcaigh
Department of Haematology, OLCHC, Crumlin, Dublin 12
Email: [email protected]
Acknowledgements
Sinead Horgan of the IUBMR for her work in sourcing the unrelated cords; Richard Hagan and the staff of the HLA typing of the National Histocompatibility; and Immunogenetics Reference Laboratory IBTS for HLA typing of patients and cords.
References
1. Dunne C, Lawlor E, O’Riordan J. (2004) ‘Probability of finding a HLA matched Sibling donor for HSCT in Ireland’. European Journal of Immunogenetics, 31:239.
2. Laughlin MJ, Eapen M, Rubinstein P, Wagner JE, Zhang MJ, Champlin RE, Stevens C, Barker JN, Gale RP, Lazarus HM, Marks DI, van Rood JJ, Scaradavou A, Horowitz MM. Outcomes after transplantation of cord blood or bone marrow from unrelated donors in adults with leukemia. NEJM. 2004;351:2265-2275.
3. Gluckman E, Broxmeyer HA, Auerbach AD, Friedman HS, Douglas GW, Devergie A, Esperou H, Thierry D, Socie G, Lehn P, Cooper S, English D, Kurtzberg J, Bard J, Boyse E. Haematopoietic reconstitution in a patient with Fanconi’s anemia by means of umbilical-cord blood from an HLA-identical sibling. NEJM. 1989;321:1174-1178.
4. Apperley J, Carreras E, Gluckman E, Gratwohl A, Masszi T. Haematopoietic Stem Cell Transplantation. The EBMT Handbook. 5th ed. 2008.
5. Gluckman E. Cord blood transplantation for children with acute leukemia: an Eurocord Registry Analysis. Biology of Blood and Marrow Transplantation. 2005;1:936-937.
6. Wagner JE, Barker JN, DeFor TE, Baker KS, Blazar BR, Eide C, Goldman A, Kersey J, Krivit W, MacMillan ML, Orchard PJ, Peters C, Weisdorf DJ, Ramsay NK, Davies SM. Transplantation of unrelated donor umbilical cord blood in 102 patients with malignant and nonmalignant diseases: influence of CD34 cell dose and HLA disparity on treatment-related mortality and survival. Blood. 2002;100:1611-1618.
7. Eapen M, Rubinstein P, Zhang MJ, Stevens C, Kurtzberg J, Scaradavou A, Loberiza FR, Champlin RE, Klein JP, Horowitz MM, Wagner JE. Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukaemia: a comparison study. Lancet. 2007;369:1947-1954.
8. Kurtzberg J, Prasad VK, Carter SL, Wagner JE, Baxter-Lower LA, Wall D, Kapoor N, Guinan EC, Feig SA, Wagner EL, Kernan NA. Results of the Cord Blood Transplantation Study (COBLT): clinical outcomes of unrelated donor umbilical cord blood transplantation in pediatric patients with hematologic malignancies. Blood. 2008;112:4318-4327. 9. Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, Thomas ED. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15:825-828.
10. National Cord Blood Program. Protocol for preparing frozen placental cord blood units for transfusion. New York Blood Center. 2006.
11. Snell M, Chau C, Hendrix D, Fox R, Downes KA, Creger R, Meyerson H, Telen MJ, Laughlin MJ, Lazarus HM, Yomtovian R. Lack of isohemagglutinin production following minor ABO incompatible unrelated HLA mismatched umbilical cord blood transplantation. Bone Marrow Transplantation. 2006;38:135-140.
12. Iori AP, Arcese W, Milano F, Calabrese E, Torelli GF, Barberi W, Mascolo MG, De Felice L, Screnci M, Lucarelli B, Malandruccolo L, Perrone MP, Salvatori S, Laurenti L, Iannella E, Ricci R, Moleti ML, Foà R. Unrelated cord blood transplant in children with high-risk acute lymphoblastic leukemia: a long-term follow-up. Haematologica. 2007;92:1051-1058.
13. Kurtzberg J, Laughlin M, Graham M, Smith C, Olson JF, Halperin EC, Ciocci G, Carrier C, Stevens CE, Rubinstein P. Placental blood as a source of hematopoietic stem cells for transplantation into unrelated recipients. NEJM. 1996;335:157-166.
14. Wagner JE, Rosenthal J, Sweetman R, Shu XO, Davies SM, Ramsay NK, McGlave PB, Sender L, Cairo MS. Successful transplantation of HLA-matched and HLA-mismatched umbilical cord blood from unrelated donors: analysis of engraftment and acute graft-versus-host disease. Blood. 1996;88:795-802.
15. Escalón MP, Komanduri KV. Cord blood transplantation: evolving strategies to improve engraftment and immune reconstitution. Curr Opin Oncol. 2010;22:122-9.