|
|
|
|
|
|
|
|
Elaine Brabazon,Micheal Carton,Gabriela Dornikova,D Bedford
|
|
|
|
Ir Med J. 2012 Jun;105(6):177-80
|
|
E Brabazon1, M Carton2, G Dornikova2, D Bedford1
1Department of Public Health, Health Service Executive, Dublin North East, Railway St, Navan, Co Meath
2Cavan General Hospital, Cavan
Abstract
Urinary tract infections (UTIs) are a major source of antimicrobial prescribing in the clinical setting and a potential reservoir for the emergence of resistant organisms. Although studies have been published on resistance rates for urinary pathogens from both hospital and general practitioner (GP) settings, there is little information from Long-Term Care Facilities (LTCFs) in Ireland. This study aimed to document the epidemiology and resistance rates in urinary isolates, in the LTCF and GP setting, from samples submitted to a typical microbiology laboratory. In 2010, there were 963 urinary isolates from LTCFs and 1,169 urinary isolates from GPs, identified from patients 65 years and over, with cytology suggestive of infection. E. coli was the most common causative organism identified. There were significantly higher levels of resistance to ampicillin, co-amoxiclav, ciprofloxacin, nitrofurantoin, trimethoprim, and piperacillin/tazobactam in the LTCF population compared to the GP population (e.g. for E. coli, 86%-v-69%; 30% -v- 21%; 58%-v-26%, 10%-v-3%, 68%-v-48%, 10% -v- 4% respectively). Isolates with resistance mechanisms to beta-lactams, were identified in both populations. Results presented in this paper demonstrate significant differences between resistance rates in LTCF and GP populations which suggest that there are implications for empiric antimicrobial prescribing for UTIs in the LTCF setting.
Introduction
The ability of pathogenic organisms to evolve mechanisms to resist antimicrobials has become a significant threat in recent years. Infection with antimicrobial resistant bacteria has been shown to increase patient morbidity and mortality, increase hospital length of stay and increase overall healthcare costs1. Inappropriate antimicrobial prescribing and inadequate infection control measures have contributed to such increases in antimicrobial resistance (AMR). Urinary tract infections (UTIs) are a major source of antimicrobial prescribing in the clinical setting and therefore a potential reservoir for the emergence of resistant organisms. The most common organism identified from UTIs is the facultative anaerobe, Escherichia coli. Resistance of this organism to ampicillin appears to be widespread in the hospital2 and general practice (GP)2,3 settings in Ireland. However, little has been documented regarding the resistance profiles of E. coli or other uropathogens in Long-Term Care Facilities (LTCFs). Recently, guidelines for antimicrobial prescribing in primary care in Ireland4 were published. However, these guidelines for empiric therapy may not be adequate in LTCFs.
The aim of this study, therefore, was to identify if there are differences in resistance rates between GP and LTCF populations using data from samples submitted to a typical microbiology laboratory, in this case a laboratory serving a population of approximately 120,000 people. This study was part of an ongoing surveillance project on antimicrobial resistance in the HSE Dublin North East region and shall provide an indication of the epidemiology of antimicrobial resistance in UTIs in the LTCF or general nursing home setting.
Methods
The microbiology laboratory information system was interrogated on 13th May 2011 to identify all culture positive isolates from urine samples submitted to the laboratory by GPs and LTCFs between 1st January 2010 and 31st December 2010. Bacteriuria was identified by only including urine samples with a predominant growth culture of greater than 10,000 colony forming units/ml and white cell count (WCC) greater than 10 cells/mm3. In order to compare between populations, only isolates from patients aged 65 years and over were included in the analysis. Patient demographics were used to identify the origin of the patient (GP or LTCF) and to produce a random unique identifier. Data were subsequently anonymised prior to analysis.
An MS Access database allowed isolate sensitivity results to be matched with patient isolate details and provided a method for aggregation of data. Aggregate data are presented for all isolates but resistance profiles are only presented on a 1st isolate per patient per year basis to reduce bias from patients that are frequently cultured or have multiple isolates of the same organism. The sensitivities reported here have been determined in the laboratory using one of a number of methodologies including manual sensitivity testing (zone of inhibition) and automated sensitivity instrument reports (BD Phoenix). Interpretations (Sensitive, Resistant, Intermediate) and analysis have been made following EUCAST5 and CLSI6 guidelines respectively and resistance rates have been rounded up as whole percentages6. Data were analysed on an annual basis to reduce complications from seasonal variation. Isolates with resistance mechanisms to various classes of antibiotics were also identified in the laboratory (e.g. ESBLs, AmpC). The organisms for which resistance mechanisms were identified were processed separately in the analysis. The two-sample t-test for comparison of proportions was used to compare percent resistance in LTCF population with GP population.
Results
Demographics
In total for 2010, there were 2,132 culture positive urinary isolates from patients aged 65 years and over identified from LTCF and GP samples. Of these, 963 urinary isolates were identified from 22 LTCFs (96.5% of which were categorised as general nursing homes). There were 1,169 urinary isolates identified from 30 GP practices. For both the GP and LTCF population, isolates from females were more common (77.8% and 68.4%) however there was no significant difference in mean age between populations (Table 1). The most common organisms isolated from urine are presented in Table 1. E. coli accounted for the largest number of isolates identified in both LTCF and GP populations (57.6% and 69.6% of all isolates respectively). When isolates were analysed by first isolate per patient per year there was a substantial drop in numbers of isolates for cumulative susceptibility profiling (i.e. analysis of resistance rates). However, this methodology eliminates bias from multiple isolates from the same patient over the study timeframe.
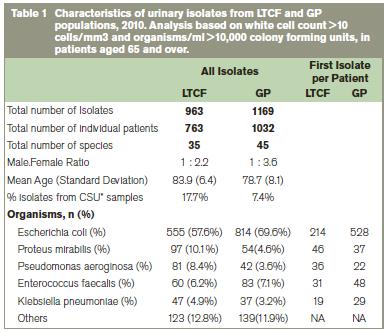
*catheter stream urine sample
Resistance Rates
Comparison of resistance rates demonstrated significant differences between LTCF and GP populations for various antibiotics. In particular, for E. coli (which accounts for a large proportion (>50%) of all isolates identified), resistance rates were statistically significantly higher for all of the major therapeutic antibiotics in the LTCF setting compared to the GP setting (Table 2). Almost all E. coli isolates from the LTCF setting were resistant to ampicillin (86%). Significantly higher levels of resistance were also demonstrated for co-amoxiclav (30% -v- 21%), ciprofloxacin (58% -v- 26%), nitrofurantoin (10% -v- 3%), trimethoprim (68% -v- 48%) and piperacillin/tazobactam (10% -v- 4%) in the LTCF population compared to the GP population. In order to assess the impact of catheterisation on resistance rates in E. coli, the analysis was also performed on mid-stream urine (MSU) specimens only. This analysis also found higher resistance rates in the LTCF population compared to the GP population for most therapeutic antibiotics which suggests that in E. coli, catheterised samples do not contribute significantly to differences in resistance between the populations. For the second most common organism identified Proteus mirabilis, significantly higher levels of resistance in the LTCF setting were shown for trimethoprim (83% -v- 38%). There were no significant differences identified in resistance rates between the two populations for P. aerugonisa, K. pneumoniae and E. faecalis isolates. However, overall numbers for these organisms were low.
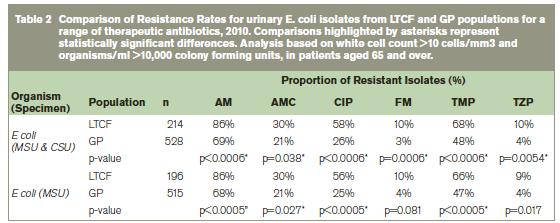
AM: ampicillin; AMC: co-amoxiclav; CIP:ciprofloxacin; FM: nitrofurantoin; TMP: trimethoprim; TZP: piperacillin/tazobactam; MSU: Mid-Stream Urine Specimen, CSU: Catheter Stream Urine Specimen
Resistance Mechanisms
A small number of urinary isolates from both populations were identified which harboured beta lactam resistance mechanisms. These included extended spectrum beta lactamases (ESBLs) and AmpC beta lactamases. ESBLs were identified from E. coli, K. pneumoniae, P. mirabilis and K. oxytoca (16 isolates from LTCFs, and 24 from GPs) translating to an overall ESBL rate of 5.4% for LTCFs and 3.8% from GPs. AmpC resistance mechanisms were identified in 14 isolates from LTCFs and 22 isolates from GPs from five species: M. morganii, E. cloacae, C. freundii, E. aerogenes and Providencia spp. There were no vancomycin resistant enterococci or methicillin resistant Staphylococcus aureus isolates identified from urine samples in either the LTCF or GP population.
Discussion
To the best of our knowledge, this paper describes for the first time a comparison of resistance rates for urinary pathogens from Irish LTCF and GP populations. Significant differences between both populations were evident. In particular, there were higher levels of resistance to antibiotics in the LTCF setting for common urinary pathogens (E. coli, P. mirabilis) than in the GP setting. Furthermore, organisms with resistance mechanisms to beta lactams, i.e. ESBL, AmpC, were also identified in both populations. High levels of resistance of LTCF and GP isolates to trimethoprim (68% and 48% respectively in E. coli) is worrying especially in light of the fact that this antibiotic is considered one of the first line treatments for uncomplicated urinary tract infection4. The contribution of both therapeutic and prophylactic use of trimethoprim to such increased resistance rates in urinary pathogens should be investigated. The other recommended antibiotic for first-line treatment of UTI, nitofurantoin, demonstrated overall low levels of resistance in E. coli in both LTCF and GP populations (10% and 3% respectively in E. coli) and would suggest that this antibiotic is a suitable therapeutic choice.
Very high levels of resistance to ciprofloxacin were seen in the LTCF setting compared to the GP setting (58% and 26% respectively in E. coli). These rates are likely to reflect concomitant high levels of prescribing of ciprofloxacin in patients7. Not only is this an issue for the evolution of fluoroquinolone-resistant organisms, high prescribing rates for ciprofloxacin have been shown to be an important risk factor for C. difficile infection8. Antimicrobial resistance profiles for urinary pathogens in various Irish populations have been previously published2,3,7,9-12 and resistance of urinary pathogens in the non acute setting to trimethoprim2,3,7,10,12 and ampicillin2,3,10,12 is evident. Most of these Irish studies on E. coli report low levels of resistance to nitrofurantoin2,3,9,10,12 and reported resistance to ciprofloxacin is similar to that reported for the GP population in this study (ranging from 1%-18%2,3,7,9-12). However, much higher levels of resistance to floroquinolones (>50%) in urine cultures have been reported internationally in the LTCF setting13,14 and there is concern about the evolution of multiple-antibiotic resistant organisms from LTCFs15.
In 2009, the Health Protection Surveillance Centre published guidelines for Antimicrobial Stewardship in Hospitals in Ireland16 which also made recommendations for non-acute residential healthcare institutions. Each facility should have an antimicrobial stewardship programme, antimicrobial audit/intervention teams and access to advice from a consultant microbiologist or infectious disease physician and antimicrobial pharmacist. There are gaps in the area of antimicrobial stewardship in LTCFs as have been highlighted by a recent Irish study17. With the documentation of high levels of resistance to common antibiotics in Irish LTCFs as described in this paper, these recommendations need to be implemented to their full extent to protect against inappropriate use of antimicrobials.
This study has provided evidence for differences in antimicrobial resistance rates for urinary isolates from GP and LTCF populations in a region in Ireland and has highlighted a worrying level of resistance to various therapeutic agents, particularly in the LTCF setting. It would seem likely, in light of the fact that catheterisation and age were not factors in the difference between rates, that exposure to antibiotics in these different settings, may significantly affect resistance rates. Such differences in rates have implications for empiric prescribing and suggest that analysis of local resistance patterns and specific antibiotic policies or guidelines for LTCFs are an essential component of any long term antibiotic stewardship plan.
Correspondence: E Brabazon
Department of Public Health, Health Service Executive, Dublin North East, Railway St, Navan, Co Meath
Email: [email protected]
Acknowledgements
We are grateful to all staff in the Microbiology Laboratory, Department of Clinical and Laboratory Sciences, Cavan & Monaghan Hospital Group and in the Department of Public Health, HSE North East for helpful discussion and advice during this project.
References
1. A Strategy for the Control of Antimicrobial Resistance in Ireland (2001). Report of the Subgroup of the Scientific Advisory Committee of the National Disease Surveillance Centre.
2. Ni Chulain M, Murray AM, Corbett-Feeney G and Cormican M. (2005) Antimicrobial resistance in E. coli associated with urinary tract infection in the West of Ireland. Irish Journal of Medical Science, 174: 8-11.
3. North Dublin SARI Newsletter, Issue 1, October 2008. Available at http://www.hpsc.ie
4. Guidelines for Antimicrobial Prescribing in Primary Care in Ireland (2011). Report produced on behalk of the SARI Community Antibioitc Stewardship Expert Working Group. Available at: http://www.hpsc.ie
5. The European Committee on Antimicrobial Susceptibility Testing, EUCAST. Details available at: http://www.eucast.org/
6. CLSI Document M39-A “Analysis and Presentation of Cumulative Antimicrobial Susceptibility Test data; Approved Guideline”
7. Vellinga A, Murphy AW, Hanahoe B, Bennett K & Cormican M. (2010) A multilevel analysis of trimethoprim and ciprofloxacin prescribing and resistance of uropathogenic Escherichia coli in general practice. Journal of Antimicrobial Chemotherapy, 65: 1514-1520.
8. Surveillance, Diagnosis and Management of Clostridium difficile – associated disease in Ireland (2008). Report by the Health Protection Surveillance Centre, Clostridium difficile Sub-Committee. Available at: http://www.hpsc.ie
9. Collins C, Baker L, Cunney R, Cafferkey M. (2011) Epidemiology and Resistance Patterns of Urinary Pathogens in Children less than Three Years Old, Irish Medical Journal, Vol 104: 27.
10. Brennan A, Murphy O, Sheahan A, O’Reilly B, Barry L, Cronin L, Kelly M, Coughlan M & O’Connell S. (2011) Abstract: Antimicrobial Resistance in Urinary Tract Pathogens in Cork & Kerry, 2004-2010. 2011 Summer Scientific Meeting 25/26th May 2011.
11. Cormican M, Morris D, Corbett-Feeney G and Flynn J. (1997) Extended spectrum beta lactamase production and fluorquinolone resistance in pathogens accociated with community acquired urinary tract infection. Diag. Microbiol Infect Dis, 32: 317-319.
12 Infoscan Communicable Disease Report, Vol 11 (4) Quarterly Edition Oct-Dec 2001. Article on Urinary Tract Infections.
13. Lautenbach E, Marsicano R, Tolomeo P, Heard M, Serrano S and Stieritz DD. (2009) Epidemiology of Gram Negative Antimicrobial Resistance in a Multi-State Network of Long Term Care Facilities. Infect. Control Hosp Epidemiol. 30: 790-793. 14. Strausbaugh LJ, Crossley KB, Nurse BA, Thrupp LD. (1996) Antimicrobial Resistance in Long Term Care Facilities. Infect. Control Hosp Epidemiol. 17: 129-140.
15. O’Fallon E, Pop-Vicas A and D’Agata E. (2009) The Emerging Threat of Multidrug Resistant Gram-Negative Organisms in Long Term Care Facilities. J Gerontol A Biol Sci Med Sci. 64A: 138-141.
16. Guidelines for Antimicrobial Stewardship in Hospitals in Ireland (2009). SARI Hospital Antimicrobial Stewardship Working Group. Available at http://www.hpsc.ie
17. European Point Prevalence Survey on Healthcare Associated Infections and Antibiotic use in Long-Term Care Facilities. National Report – Republic of Ireland, November 2010 & September 2011. Available at: http://www.hpsc.ie
|
|
|
|
Author's Correspondence
|
|
No Author Comments
|
|
|
Acknowledgement
|
|
No Acknowledgement
|
|
|
Other References
|
|
No Other References
|
|
|
|
|