|
|
|
|
|
|
|
|
Darren Mullane,Christopher McGuigan,Aine Merwick,Laura Williams,AM Tobin
|
|
|
Ir Med J. 2012 Jun;105(6):182-3
D Mullane, L Williams, A Merwick, WO Tobin, C McGuigan
Department of Neurology, St Vincent’s University Hospital, Elm Park, Dublin 4
Abstract
Drug induced aseptic meningitis (DIAM) is an uncommon condition that can mimic infective conditions. DIAM has been recognized with various treatments including non-steroidal anti-inflammatory drugs, monoclonal antibodies and some antibiotics. We report a patient presenting with aseptic meningitis forty-eight hours after commencing a course of intravenous immunoglobulin (IVIG) treatment. It is important that physicians prescribing this medication are aware of this rare complication so the diagnosis can be made quickly and the patient is not exposed to unnecessary treatments.
|
Case Report
A thirty-five year old woman with a history of acetylcholine receptor (ACHR) antibody positive Myasthenia Gravis (MG) was commenced on a five day course of IVIG for worsening myasthenic symptoms at a dose of 4g/Kg/day. Of note she had not commenced any new treatment prior to this therapy but was maintained on a low dose of azathioprine. The patient developed a mild headache following the second infusion but did not have any associated symptoms. The following morning she experienced a sudden onset vertex headache that she rated at 10/10 in severity. The headache was aggravated by movement and associated with nausea and vomiting. The patient did not have sensitivity to light or noise. She had a history of migraine headaches without aura but described the current headache as differing in quality to her usual headache.
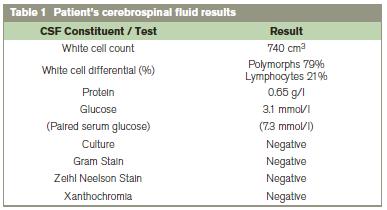
On examination she was apyrexial and did not exhibit neck stiffness or photophobia. Physical examination revealed signs consistent with myasthenia gravis but no new focal abnormalities were identified. The patient did not have any skin rashes. Full blood count, urea, electrolytes and inflammatory markers were all normal. A CT scan and contrast enhanced MRI scan of her brain (Figure 1) were normal. Given the sudden onset and severity of the headache a lumbar puncture examination was obtained. This revealed an elevated white Cell Count of 740/Cmm3 (79%Neutrophils). CSF protein was mildly elevated and CSF glucose reduced relative to serum. Xanthochromia was not detected (Table 1). A viral agent was considered unlikely given the CSF constituents and temporal relationship of the symptoms to IVIG therapy. The patient was diagnosed with aseptic meningitis secondary to IVIG and treated with regular analgesia and hydration. This woman was discharged with a marked improvement in symptoms and a follow up Lumbar Puncture one month later was acellular.
Figure 1: Contrast enhanced T1 saggital MRI scan of brain showing no evidence of gross meningeal thickening or abnormal contrast enhancement.
Discussion
This case highlights the need to be aware of, and vigilant for, aseptic meningitis as a drug induced complication of IVIG. Drug induced aseptic meningitis has been recognized as a complication of treatment with various agents including NSAIDS, monoclonal antibodies and antibiotics in addition to IVIG.1 Intravenous immunoglobulins are commonly used for a host of autoimmune and related conditions. The more commonly described adverse events include headache, nausea, vomiting and flushing, which are reported by 1-15% of patients receiving the therapy.2 Aseptic meningitis as a complication of IVIG treatment is uncommon. Reports in the literature suggest a prevalence of approximately 1%.3-5 However this could be an underrepresentation of the true incidence as many patients experiencing headaches with the treatment do not have further investigations including lumbar puncture. One retrospective analysis of a prospective cohort study of 54 patients found clinical evidence of aseptic meningitis in 11% of patients following IVIG.6 The factors, which may predispose to the development of the condition, include fast infusion rates and a history of migraine headaches6 as seen in this case. The symptoms of aseptic meningitis generally occur within 48 hours of starting treatment but can present up to seven days following the last infusion.6 The cause of aseptic meningitis with IVIG is unknown but theories have included an allergic hypersensitivity reaction or serum immunoglobulin crossing the blood brain barrier.6
The management of the condition is supportive with analgesia for symptom relief. If there is no evidence of an infective process antibiotics are not required. Patients make an excellent recovery and there is evidence that IVIG can be administered in the future if necessary at a slower infusion rate and with anti-histamine co-administration.7 Azathioprine, has been associated with aseptic meningitis, particularly in the context of patients with systemic lupus erythematosis (SLE)8, but symptoms usually occur within 48 hours of commencing the drug and responds to drug withdrawal making it an unlikely cause in this case.
Correspondence: C McGuigan
Department of Neurology, St Vincent’s University Hospital, Elm Park, Dublin 4
Email:[email protected]
References
1. G Moris & C Garcia-Monco. The Challenge of Drug-Induced Aseptic Meningitis. Arch Intern Med 1999;159:1185-1194
2. Orbach H, Katz U, Sherer Y, Shoenfeld Y. Intravenous immunoglobulin: adverse effects and safe administration, Clin Rev Allergy Immunol 2005;29:173–184
3. Sherer Y, Levy Y, Langevitz P, Rauova L, Fabrizzi F, Shoenfeld. Adverse effects of intravenous immunoglobulin therapy in 56 patients with autoimmune diseases (2001), Pharmacology 2001;62:133–137
4. Brannagan TH, Nagle KJ, Lange DJ, Rowland LP. Complications of IVIG treatment in neurological disease. Neurology 1996;47:674–677
5. Stangel M, Kiefer R, Pette M, Smolka MN, Marx P, Gold R. Side effects of intravenous immunoglobulins in neurological autoimmune disorders – a prospective study. J Neurol 2003;250:818–821.
6. Sekul EA, Cupler EJ, Dalakas MC. Aseptic meningitis associated with high-dose intravenous immunoglobulin therapy: frequency and risk factors, Annals of Internal Medicine 1994;121:259–262
7. S Jolles & H Hill. Management of aseptic meningitis secondary to intravenous immunoglobulin. BMJ 1998;316:936
8. Sergent JS & Lockshin M. Azathioprine-induced meningitis in systemic lupus erythematosus. JAMA 1978;240:529
|
|
|
|
Author's Correspondence
|
|
No Author Comments
|
|
|
Acknowledgement
|
|
No Acknowledgement
|
|
|
Other References
|
|
No Other References
|
|
|
|
|