|
|
|
|
|
|
|
|
CP Hawkes,Stephanie Mulcair,JO'B Hourihane
|
|
|
|
Ir Med J. 2010 Jan;103(1):17-9.
|
CP Hawkes, S Mulcair, JOB Hourihane
Department of Paediatrics and Child Health, UCC, Immunisation Clinic, Cork University Hospital, Wilton, Cork
Abstract
Egg allergy is incorrectly considered to constitute a contraindication to MMR in the community, despite a long history of its safe administration to egg allergic children. The product insert perpetuates this misinformation but the Irish guidelines from the RCPI are unequivocal. We reviewed all paediatric cases vaccinated in our hospital in 2007-2008. Forty seven of 91 children receiving vaccinations in hospital, had been referred for MMR due to concerns regarding egg allergy. In 32% (n=15), GP referral for vaccination was made despite correspondence from the clinic advising routine vaccination in the community. Nineteen were second MMR immunisations, which should all have occurred in the community. Unnecessary hospital referral for MMR vaccination is an extra burden on hospital resources, and causes unwarranted anxiety amongst parents of children with egg allergy. A change in practice seems difficult to achieve, as many referrals happen despite individualised correspondence to GPs and other referring clinicians outlining the current guidelines.
Introduction
Egg allergy affects 1.5% to 3.2% of children1 and, of those, 66% will have outgrown it by the age of 52. Current recommendations are that all children with egg allergy are followed with serial specific IgE levels, skin prick testing and, when appropriate, hospital based food challenge in order to assess whether tolerance has developed1. Egg allergy is often, incorrectly, thought to constitute a contraindication for measles, mumps and rubella vaccination (MMR). The measles vaccine is produced in a culture of chick embryo fibroblasts and it was shown nearly 40 years ago that it can contain up to 0.001mcg of ovalbumin3,4. This is 2 million times lower than the minimal oral dose of allergen required to elicit a reaction in double blind placebo controlled trials of peanut allergy5, which is generally a more severe type of food allergy than egg allergy. Even when delivered via the parenteral route, this dose of ovalbumin is likely to be suballergenic.
Allergenic components present in larger quantities in the vaccine, such as gelatin or neomycin, are more likely to be the cause of the extremely rare allergic reactions that may occur with this vaccine6,7. Pool et al performed a case-control study involving children with anaphylaxis post MMR, with children who tolerated the MMR as controls. They found that the specific IgE to gelatin was significantly higher in the anaphylactic group, with no significant difference in specific IgE to egg. One quarter of children with anaphylaxis had hypersensitivity to gelatin7. The occurrence of anaphylaxis in children with egg allergy following MMR is extremely rare, and, because children without egg allergy are at a similar risk (1 in 200,000 to 1 in 1,000,000), referral for vaccination in hospital is not usually indicated. Despite the long history of safe provision of MMR for children with egg allergy, the manufacturers have been slow to advocate its use in this scenario. Merck state that "persons with a history of anaphylactic, anaphylactoid or other immediate reactions (e.g., hives, swelling of the mouth and throat, difficulty breathing, hypotension, or shock)" following egg ingestion "may be at an enhanced risk". Confusion can also result from use of the words "anaphylactoid" and "anaphylactic" beside "hives" and "swelling", which are indicative of allergic but not automatically anaphylactic reactions. They also state that the "potential risk to benefit ratio should be carefully evaluated before considering vaccination in such cases"8. GlaxoSmithKline and Sanofi have similar disclaimers in their product literature.
The Royal College of Physicians of Ireland states that "recent data suggest that anaphylactic reactions to MMR are not associated with hypersensitivity to egg antigens"9. The British Society for Allergy and Clinical Immunology (BSACI) guidelines state that in children with known egg allergy, MMR can safely be provided in the community. As with all vaccination, adrenaline should be readily available as there is always a very small risk of anaphylaxis. Khakoo et al suggest that hospital MMR may be appropriate in children with egg allergy and a history of anaphylaxis or chronic severe asthma10. This recommendation is based on the fact that these children are likely to have a severe reaction to egg if they react to MMR (which is unlikely), not when they react to MMR. Most paediatric allergists follow this pragmatic guideline. Cork University Hospital has a part time immunisation nurse specialist, who receives referral for hospital MMR directly from general practitioners (GP) and Area Medical Officers (AMO). A weekly specialist allergy clinic runs separately to this service. The purpose of our study is to review the children with egg allergy referred for the MMR to be provided in hospital. We also aim to determine if those children with egg allergy causing sufficient anxiety to be referred to hospital for MMR are also being referred to the allergy clinic.
Methods
Details of referrals for hospital delivered vaccination in 2007 and 2008 were prospectively collected. Those children with egg allergy were identified and their demographic details were recorded. This list was then cross checked with the prospectively collected details of paediatric allergy clinic attendees. Assessment of egg allergic children in the allergy clinic includes suitability for community immunisation and explicit advice is given in the clinic letter to the referring doctor.
Results
Over a two year period from 1/1/07 to 31/12/08, 91 children were referred to hospital for vaccination. Egg allergy was noted as the indication in 47 children (20 in 2007, 27 in 2008). The nature of reaction to egg was not recorded for 4 children whose charts could not be located. Vaccination was provided at a mean age of 2.8 years for first MMR. Delay in age of second MMR could not be quantified as there was a catch up programme in place, and children could be referred at 4-5 years (pre-school) or 11-12 years of age (leaving primary school). The median time from receipt of referral to vaccination was 37 days (Interquartile Range=22-75). No adverse reactions to vaccination were reported.
Figure 1: Referral Pathways for Hospital Vaccination
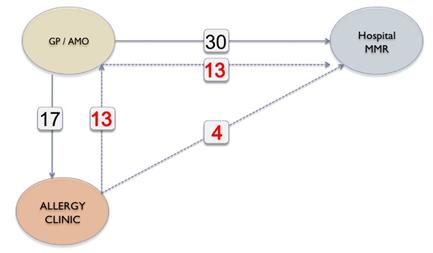
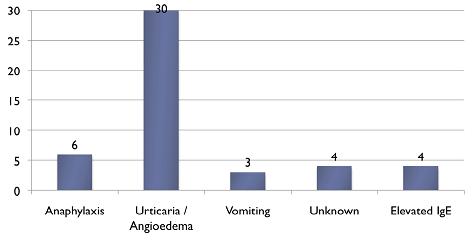
Twenty five referrals were for first MMR, while 19 were for second MMR. In all children receiving their second MMR in hospital, the first MMR had been provided without complications, so these were all inappropriate re-referrals. Seventeen children were seen in the allergy clinic and 4 of those were referred for hospital MMR due to a history of anaphylaxis. Thirteen children referred to the allergy clinic were considered suitable for community administration of MMR but all were subsequently referred by the AMO or GP for MMR in hospital (Figure 1). In 2 further cases, referral for hospital MMR was made despite direct correspondence from the immunisation clinic stating that vaccination in the community was more appropriate. Thus, there had been communication with GPs outlining the safety of MMR in the community for 15 (32%) of the children subsequently referred. Four children were unnecessarily referred to hospital for MMR on the basis of an elevated IgE or RAST test for egg, in the absence of a documented reaction to egg (Figure 2). Two children referred, with a history of anaphylaxis to egg, were assessed safe for community immunisation as they had safely received their first MMR without complication.
Figure 2: Nature of Egg Reaction
Discussion
Egg allergy affects up to 2% of young children, and the provision of MMR for this population can be a source of anxiety in the community. This is largely perpetuated by poorly written product literature, which may incorrectly cause physicians and parents not to vaccinate their child. All three MMR manufacturers have a disclaimer that resembles a "may contain allergen" label, which puts the burden of responsibility onto the parents and physician. The immunisation information booklet given to all parents in Ireland states that the "MMR vaccine can be given to children with a severe egg allergy, as severe allergy to the MMR vaccine is extremely rare even in these children"11. This contrasts with the product literature. Such conflict can be a source of confusion for parents.
While current best evidence recommends the provision of MMR in the community in most cases, there is a resistance to adopting this approach. We found that correspondence directly from the allergy or immunisation clinics for 15 (32%) of the children referred did not result in the provision of MMR in the community. This is a problem that may be difficult to correct due to common custom and practice in the community over the past twenty years. Failure to vaccinate children in the community puts an unnecessary burden on hospital resources. Furthermore, the MMR already has a controversial history as many parents still believe in the disproven link between MMR and autism. If an uncertain parent is told by a physician that the child will need to have the MMR in hospital because of a possible reaction, the vaccine may not be provided in a timely fashion (our data shown a mean age of first MMR of 2.8 years) and possibly not at all. This increases the risk of wild disease.
In this study, we have only studied those children whose parents had agreed to hospital MMR, so it is very likely that other children with egg allergy are not being vaccinated against MMR at all. Of note we did not include children with egg allergy attending our clinic, who received the MMR in the community. There may also be children referred, who following communication between the allergy clinic and GP or AMO, subsequently receive the vaccination in the community. Thus, we have not quantified fully the compliance with current guidelines that may exist, but we have highlighted a degree of resistance. We acknowledge that this resistance is not universal in the community. The allergy clinic has only existed for 3 years and the issue was dealt with differently before its establishment. In this study, 4 children were referred from the community for hospital MMR on the basis of a positive specific IgE for egg, in the absence of a reaction to egg. This is a common misconception amongst physicians. An elevated specific IgE indicates sensitisation to the allergen, it does not indicate clinical allergy. Serum based tests alone cannot confirm egg allergy, and many of these children may have been unnecessarily avoiding egg12. The allergy clinic allows accurate diagnosis and risk assessments to be made. However, egg allergy is a transient phenomenon for many children. The allergy clinic can follow these children with serial IgE and skin prick tests, in order to optimally time a hospital based challenge and possible reintroduction of egg to the diet. Advice on egg product avoidance, as well as the provision of adrenaline pens and training can also be provided by the allergy clinic. Unfortunately, 64% of children referred for hospital MMR were not referred to the clinic and were not availing of this service. This highlights suboptimal management of this condition in the community.
Following this audit, we have implemented a number of changes in our practice. All letters of referral to the immunisation clinic are now reviewed by a consultant. A copy of the BSACI advice is sent to all GPs who refer patients for MMR in hospital with a letter offering to discuss each case by phone. A notice was also placed in the quarterly Public Health Bulletin, received by all local GPs and AMOs. This stated that children with allergies should be referred to the allergy clinic for assessment, and that routine immunisation should proceed unless there is a genuine contraindication. We suggest that GPs and AMOs review their current immunisation practices regarding children with egg allergy who are nearly always eligible for MMR vaccination in the community. Awareness of the evidence surrounding this topic is necessary to confidently reassure parents prior to delivery of this vaccine. The product insert is misleading, and can make the process of reassurance more difficult. We suggest that manufacturers rephrase the product insert after consultation with experienced allergists and not just with lawyers.
Correspondence: JOB Hourihane
Department of Paediatrics and Child Health, UCC, Immunisation Clinic, Cork University Hospital, Wilton, Cork
Email: [email protected]
References
1. Lack G. Clinical practice. Food allergy. N Engl J Med. 2008 Sep 18;359:1252-60.
2. Allen CW, Campbell DE, Kemp AS. Egg allergy: are all childhood food allergies the same? J Paediatr Child Health. 2007 Apr;43:214-8.
3. O'Brien T, Maloney C, Tauraso N. Quantitation of residual host protein in chicken embryo-derived vaccines by radial immunodiffusion. Appl Microbiol. 1971;21:780-2.
4. Fasano MB, Wood RA, Cooke SK, Sampson HA. Egg hypersensitivity and adverse reactions to measles, mumps, and rubella vaccine. J Pediatr. 1992 Jun;120:878-81.
5. Hourihane JO’B, Kilburn SA, Nordlee JA, Hefle SL, Taylor SL, Warner JO. An evaluation of the sensitivity of subjects with peanut allergy to very low doses of peanut protein: a randomized, double-blind, placebo-controlled food challenge study. J Allergy Clin Immunol. 1997 Nov;100:596-600.
6. Sakaguchi M, Ogura H, Inouye S. IgE antibody to gelatin in children with immediate-type reactions to measles and mumps vaccines. J Allergy Clin Immunol. 1995 Oct;96:563-5.
7. Pool V, Braun MM, Kelso JM, Mootrey G, Chen RT, Yunginger JW, et al. Prevalence of anti-gelatin IgE antibodies in people with anaphylaxis after measles-mumps rubella vaccine in the United States. Pediatrics. 2002 Dec;110:e71.
8. Johansson SG, Bieber T, Dahl R et al. Revised nomenclature for allergy for global use: Report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J Allergy Clin Immunol. 2004 May;113:832-6.
9. Khakoo GA, Lack G. Recommendations for using MMR vaccine in children allergic to eggs. BMJ. 2000 Apr 1;320:929-32.
10. Health Service Executive Ireland. Your Child's Immunisation - A guide for parents. April 2008:18.
11. Elliman D, Bedford H. MMR vaccine: the continuing saga. BMJ. 2001 Jan 27;322:183-4.
12. Cox L, Williams B, Sicherer S et al. Pearls and pitfalls of allergy diagnostic testing: report from the American College of Allergy, Asthma and Immunology/American Academy of Allergy, Asthma and Immunology Specific IgE Test Task Force. Ann Allergy Asthma Immunol. 2008 Dec;101:580-92.
|
|
|
|
Author's Correspondence
|
|
No Author Comments
|
|
|
Acknowledgement
|
|
No Acknowledgement
|
|
|
Other References
|
|
No Other References
|
|
|
|
|