|
|
|
|
|
|
|
|
APH Walsh,Lyuda V Shkrobot,Graham D Coull,Kelli L Peirce,DJ Walsh,Umme Salma,E Scott Sills
|
|
|
|
Ir Med J. 2009 Oct;102(9):282-5.
|
|
APH Walsh, LV Shkrobot, GD Coull, KL Peirce, DJ Walsh, U Salma, ES Sills
The Sims Institute/Sims International Fertility Clinic, Rosemount Hall, Dundrum Road, Dublin 14
Abstract
Patients with recurrent IVF failure are generally regarded as having a poor prognosis, and when female age exceeds 35yrs such patients face a particularly bleak outlook. This study reported on blastocyst transfer (BT) performed over a five-year interval in patients seeking “second opinion” after multiple failed IVF cycles. Clinical features and reproductive outcomes were compared between two sets of poor-prognosis IVF patients undergoing BT for the first time, the initial group underwent treatment in 2002 (n=66) and a second group presented five years later (n=392). The two clinical sets had no patients in common. The 2002 group had an average of 3.5(±1.1) prior failed IVF cycles at baseline, and mean (±SD) patient age was 36.4(±3.9)yrs. Average number of oocytes retrieved in this group was 10.4(±5.3) with a fertilisation rate of 58.8%. Although embryo arrest resulted in no transfer for 19 patients (28.8%), clinical pregnancy was achieved for 59.6% of transfers. Five years later, 392 patients underwent BT, but this group had an average of 4.5(±2.3) prior failed IVF cycles. Mean (±SD) female age was 36.0(±3.9)yrs, and the average number of oocytes retrieved in this group was 9.1(±5.4); the fertilisation rate was 59.5%. No blastocysts were available for transfer in 99 cases (25.3%); clinical pregnancy was achieved for 50.0% of transfers. The number of blastocysts transferred was similar in the two groups (1.6 vs. 1.3; p=0.06); the twinning rate rose slightly from 8.2% to 15.1% (p=0.12) despite an increased utilisation of single embryo transfer in 2007 (19.7% vs. 22.2%; p=0.40). Comparisons from 2002 and 2007 found no important differences between the two patient groups, except for a significantly higher rate of prior failed cycles in the 2007 group (p<0.001). This refractoriness was accompanied by a somewhat reduced blastocyst cryopreservation rate in 2007, compared to 2002 (27.6% vs. 29.5%; p=0.44). Clinical pregnancy rates are not adversely affected by application of BT in patients with multiple prior unsuccessful IVF cycles. For these patients, our data suggest that extended embryo culture and BT should be considered. Further controlled studies are needed to document more precisely the role of BT in this sub-set of refractory IVF patients.
Introduction
Extended in vitro embryo culture and blastocyst transfer (BT) are now established components of the advanced reproductive technologies, permitting selection of more advanced embryos considered best suited for transfer. In the years since the first successful IVF, the optimal time to perform embryo transfer (ET) has remained a mystery. Historically, cleavage stage (day 2 or 3) ET was used first in IVF and therefore became established as the usual laboratory approach. This intentional placement of a day 2 or 3 embryo directly into the uterine cavity was recognised as non-physiologic, but it was not immediately possible to culture such embryos until they reached the blastocyst stage for transfer. Accordingly, the traditional “choice” to perform day 2 or 3 ET represented a practical default in response to substantial technical limitations associated with extended in vitro culture. However, with the advent of more sophisticated sequential media, the potential for BT became a reality. Although the benefits of BT have been widely debated1-3, the reality of clinical practice is that not all patients are proper candidates for BT since it is possible that after five days in culture, no embryo will develop into a blastocyst resulting in cancellation of the IVF cycle. Selection criteria for BT are quite variable and there is no consensus on the appropriateness of BT protocols applied specifically to patients with multiple unsuccessful IVF cycles. Accordingly, we sought to investigate the impact of increased refractoriness to IVF on reproductive outcome following BT in two patient groups with a history of repetitive failed day three embryo transfers.
Methods
Patient selection and pre-treatment counselling
All study patients initiated IVF cycles either in 2002 or 2007 (but not both); each patient had ≥2 prior treatment cycles utilising day 3 ET resulting either in miscarriage or no pregnancy, similar to criteria described previously4. A pilot BT programme was developed at our institution in 2002 reserved for patients referred for multiple treatment failure. The second study group underwent BT in 2007 using the same inclusion criteria. Written informed consent was obtained and a review of all medical records including laboratory data and embryology worksheets was performed before treatment commenced. A focused physical examination and saline infusion sonogram to assess endometrial contours were completed, and controlled ovarian hyperstimulation regimens were developed from factors including historical response to medications, patient age, BMI and ovarian reserve assessment. At entry, couples were counselled about outcomes and likelihood of IVF failure. From this, they expressed an understanding of the role of BT as a step along their fertility journey which might ultimately include donor oocyte IVF, or perhaps to cease fertility treatments altogether. Formal psychological counselling resources were offered to all couples.
Controlled ovarian hyperstimulation
Pituitary downregulation was achieved with oral contraceptives and GnRH agonist, followed by daily administration of gonadotropins using a combined FSH+hMG protocol. Dosing levels varied and were based on prior response to gonadotropins; titration was influenced by real-time assessment of follicular development as judged by transvaginal sonogram and/or serum oestradiol data. Treatment continued until adequate ovarian response was attained, defined as the maximum potential number of follicles with mean diameter =17mm. Transvaginal sonogram-guided oocyte retrieval was accomplished 36h after subcutaneous administration of hCG.
Blastocyst culture protocol
Immediately after retrieval oocyte-cumulus complexes were placed into Universal IVF medium (MediCult; Jyllinge, Denmark), with insemination (including ICSI) also carried out using this reagent under washed liquid paraffin oil (MediCult, Denmark). Fertilisation was assessed after 16-18h and was considered normal when two distinct pronuclei were noted. Culture was maintained to day five in microdrops of BlastAssist media I and II (MediCult, Denmark) under washed paraffin oil in a 5%CO2 + 5%O2 atmosphere at 95% humidity. Embryos were assessed daily for cell number, degree of fragmentation, and compaction. Day five blastocysts selected for in utero transfer generally demonstrated a well-defined inner cell mass and highly cellular, expanding trophoectoderm. Blastocysts were then loaded into an embryo transfer catheter (K-Soft-5000 Catheter; Cook Medical Inc., Spencer, Indiana USA). All transfers occurred no earlier than 120h post-fertilisation and were uniformly carried out under direct transabdominal sonogram guidance by a physician.
Measured parameters and statistical analysis
The following factors were recorded and compared between the 2002 and 2007 BT groups: 1) patient age, 2) number of failed prior IVF (d3 ET) cycles, 3) total number of oocytes retrieved, 4) method of fertilisation [i.e., ICSI vs. conventional insemination], 5) fertilisation rate (%2pn), 6) frequency of cycle cancellation due to non-availability of blastocysts, 7) number of blastocysts transferred, 8) application of single blastocyst transfer, 9) clinical pregnancy (including frequency of multiple gestation outcomes). Due to some variation in gonadotropin formulation across patients during the five-year assessment period, and since not all patients had regular intracycle serum oestradiol measurements, these parameters were not separately studied. Student’s t-test was used for analysis, as appropriate. Differences measured at p<0.05 were considered significant.
Results
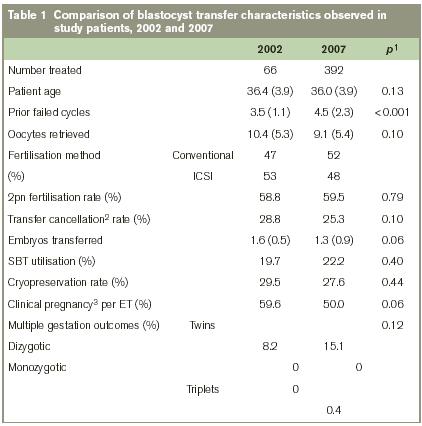
In the initial group of BT patients (n=66) treated in 2002, an average of 3.5 prior failed cycles per patient at baseline was reported. Mean age was 36.4yrs in this group. Although the mean patient age remained similar between the 2002 and the 2007 groups (p=0.13), the average number of failed IVF cycles/patient reported at baseline was significantly different (3.5 in 2002 vs. 4.5 in 2007; p<0.001). The mean number of oocytes retrieved was also lower in 2007 compared to 2002, but this decrease did not reach statistical significance (p=0.10). For 2002 and 2007, the proportion of IVF cycles incorporating intracytoplasmic sperm injection (ICSI) was almost identical, and the rate of 2pn fertilisation in these two groups was also similar (58.8% in 2002 vs. 59.5% in 2007; p=0.79). Comparing 2002 to 2007, there was a trend towards fewer IVF cycle cancellations due to cellular arrest (i.e., no blastocysts available) and no transfer (28.8% in 2002 vs. 25.3% in 2007), although this reduction was not significant (p=0.10). Mean number of embryos transferred per patient was reduced during this five year interval; indeed, single embryo transfer was applied more often in 2007 than in 2002, although neither of these changes reached statistical significance. Multiple gestation trends showed no significant change in the two study groups, and no monozygotic twins resulted from treatment in either group. A summary of clinical and laboratory features for our patients undergoing BT in 2002 and 2007 is given in Table 1.
Discussion
Patients not conceiving after several IVF attempts typically face a difficult prognosis, often accompanied by substantial emotional and financial drain. How best to guide medical decisions about recurrent IVF failure remains the subject of considerable debate, and is characterised by highly varied assessments and treatment modalities5. Diagnostic strategies generally include studies of maternal endocrine, anatomic, immunologic, infectious, and genetic parameters6. While oocyte quality has been regarded as an important element in recurrent IVF failure7, the way follicular recruitment protocols influence this remains difficult to measure and verify. For example, it has been proposed that adjustments to controlled hyperstimulation regimens in IVF might reduce embryo fragmentation and optimise gamete quality8. There are no controlled studies to prove this however4, and our earlier comparisons of different IVF stimulation regimens did not reveal any significant impact on pregnancy rate9,10.
Attention has also been focused on the genetic integrity of the embryo as the extent of chromosomal abnormality is likely higher in embryos from patients experiencing multiple IVF failures11. Impaired implantation associated with embryo aneuploidy led to implantation failure being recognised as the most frequent cause of unsuccessful IVF, rendering blastocyst nidation the key rate-limiting step in the pregnancy equation12,13. For example, when pre-implantation genetic diagnosis (PGD) was applied to embryos from a population of patients with recurrent IVF failure, aneuploidy was noted more frequently in the cycle that followed the first failure14, suggesting a reduced capacity to produce “high quality” embryos among patients with recurrent IVF failure15. Yet for patients undergoing IVF in Ireland, any practical clinical role for PGD is complicated by legal and ethical issues regarding its provision here16. Concerns have also been raised about the paucity of robust evidence supporting a beneficial effect of embryo biopsy in the setting of multiple IVF failures17. It was against this background that BT has become positioned as an alternative to human embryo biopsy and PGD, potentially serving a helpful role in the management of recurrent IVF failure18. However, the impact of increased IVF refractoriness on the rationale to maintain BT in the treatment armamentarium for such patients remains poorly defined.
To answer this, we gathered data from the first clinical blastocyst programme developed in Ireland to identify two populations that were fairly homogenous, except for the number of failed IVF cycles at baseline (before BT treatment here). Data from this institution was collected from 2002 and 2007 to accomplish this. None of these patients had ever had BT before, although they had all undergone multiple (failed) IVF cycles incorporating d3 ET. While the average patient age remained fairly consistent, those in the 2007 series did have significantly more prior failed IVF cycles at baseline, compared to the 2002 group (3.5 in 2002 vs. 4.5 in 2007; p<0.001). We also noted a trend that fewer oocytes were retrieved per patient in 2007 compared to 2002, accompanied by an impairment in blastocyst cryopreservation during this same interval (29.5% vs. 27.6%; p=0.44). Nevertheless, these refractory tendencies in the 2007 group did not have a significantly negative impact on overall reproductive outcome. Several factors could explain these findings. For example, it may be that expertise with blastocyst reagents and/or transfer techniques improved over time, and this adaptation contributed to the nearly six-fold jump in BT observed between 2002 and 2007. Interestingly, this was achieved with fewer embryos being transferred per patient, on average, from 2002 to 2007. While not a statistically important reduction, the lower embryo transfer/patient numbers in 2007 might also reflect enhancements of embryo culture and represents a subject of further investigation. This is a relevant arena for research, particularly since the multiple gestation rate trended upward (8.2% vs. 15.1%; p=0.12) despite enhanced utilisation of single blastocyst transfer in 2007.
Concerns have recently emerged regarding a potential association between extended embryo culture (i.e., longer exposure to laboratory reagents) and imprinting or epigenetic mutations in offspring conceived after blastocyst culture and transfer19. While the mechanism(s) by which a particular culture milieu may affect such mutations remains unknown, we agree with the most current ASRM report on extended culture in IVF20 supporting standardisation of laboratory conditions and monitoring the health of infants conceived from this technology. Some limitations in this descriptive report must be acknowledged. Our sample was self-selected and confined only to BT patients; it did not include a matched control group undergoing d3 ET. Although d3 ET was offered to simplify treatment and to reduce cost, randomisation was not possible because these patients all declined another d3 ET (perhaps because d3 ET was identified with the earlier IVF failures). Moreover, it is not known what proportion of these patients might have conceived if they had undergone another d3 ET. Additionally, since our IVF centre is independent from a maternity hospital, collecting delivery data is a function of voluntary reporting from obstetricians and/or patients remote from our unit, making this information difficult to gather systematically.
In conclusion, this report validates a role for BT for patients with multiple failed IVF cycles where d3 ET had been performed previously. A truly comparative investigation of d3 ET vs. BT in similar patients (where all other conditions are controlled), while ideal, is difficult to construct. It will be important to undertake further research in our population to better define which patients are best suited for BT, and this represents the focus of ongoing studies.
Correspondence: ES Sills
The Sims Institute/Sims International Fertility Clinic, Rosemount Hall, Dundrum Road, Dublin 14
Email: [email protected]
References
1. Edwards RG, Beard HK. Is the success of human IVF more a matter of genetics and evolution than growing blastocysts? Hum Reprod 1999;14:1-4.
2. Alper MM, Brinsden P, Fischer R, Wikland M. To blastocyst or not to blastocyst? That is the question. Hum Reprod 2001;16:617-9.
3. Hartshorne GM, Lilford RJ. Different perspectives of patients and health care professionals on the potential benefits and risks of blastocyst culture and multiple embryo transfer. Hum Reprod 2002;17:1023-30.
4. Margalioth EJ, Ben-Chetrit A, Gal M, Eldar-Geva T. Mini-review—Developments in Reproductive Medicine. Investigation and treatment of repeated implantation failure following IVF-ET. Hum Reprod 2006;21:3036-43.
5. Tan BK, Vandekerckhove P, Kennedy R, Keay SD. Investigation and current management of recurrent IVF treatment failure in the UK. BJOG 2005;112:773-80.
6. Christiansen OB, Nielsen HS, Kolte AM. Future directions of failed implantation and recurrent miscarriage research. Reprod Biomed Online 2006;13:71-83.
7. Levi Setti PE, Colombo GV, Savasi V, Bulletti C, Albani E, Ferrazzi E. Implantation failure in assisted reproduction technology and a critical approach to treatment. Ann N Y Acad Sci 2004;1034:184-99.
8. Scott L. Embryological strategies for overcoming recurrent assisted reproductive technology treatment failure. Hum Fertil (Camb) 2002;5:206-14.
9. Sills ES, Schattman GL, Veeck LL, Liu HC, Prasad M, Rosenwaks Z. Characteristics of consecutive in vitro fertilization cycles among patients treated with follicle-stimulating hormone (FSH) and human menopausal gonadotropin versus FSH alone. Fertil Steril 1998;69:831-5.
10. Sills ES, Levy DP, Moomjy M, McGee M, Rosenwaks Z. A prospective, randomized comparison of ovulation induction using highly purified follicle-stimulating hormone alone and with recombinant human luteinizing hormone in in-vitro fertilization. Hum Reprod 1999;14:2230-5.
11. Voullaire L, Collins V, Callaghan T, McBain J, Williamson R, Wilton L. High incidence of complex chromosome abnormality in cleavage embryos from patients with repeated implantation failure. Fertil Steril 2007;87:1053-8.
12. Boomsma CM, Macklon NS. Does glucocorticoid therapy in the peri-implantation period have an impact on IVF outcomes? Curr Opin Obstet Gynecol 2008;20:249-56.
13. Goodman C, Jeyendran RS, Coulam CB. Vascular endothelial growth factor gene polymorphism and implantation failure. Reprod Biomed Online 2008;16:720-3.
14. Pagidas K, Ying Y, Keefe D. Predictive value of preimplantation genetic diagnosis for aneuploidy screening in repeated IVF-ET cycles among women with recurrent implantation failure. J Assist Reprod Genet 2008;25:103-6.
15. Farhi J, Ben-Haroush A, Dresler H, Pinkas H, Sapir O, Fisch B. Male factor infertility, low fertilisation rate following ICS.I and low number of high-quality embryos are associated with high order recurrent implantation failure in young IVF patients. Acta Obstet Gynecol Scand 2008;87:76-80.
16. Soini S. Preimplantation genetic diagnosis (PGD) in Europe: diversity of legislation a challenge to the community and its citizens. Med Law 2007;26:309-23.
17. Anderson RA, Pickering S. The current status of preimplantation genetic screening: British Fertility Society Policy and Practice Guidelines. Hum Fertil (Camb) 2008;11:71-5.
18. Barrenetxea G, López de Larruzea A, Ganzabal T, Jiménez R, Carbonero K, Mandiola M. Blastocyst culture after repeated failure of cleavage-stage embryo transfers: a comparison of day 5 and day 6 transfers. Fertil Steril 2005;83:49-53.
19. Manipalviratn S, DeCherney A, Segars J. Imprinting disorders and assisted reproductive technology. Fertil Steril 2009;91:305-15.
20. ASRM/SART. Blastocyst culture and transfer in clinical assisted reproduction [Practice committee report]. Fertil Steril 2008;90:S174-7.
|
|
|
|
Author's Correspondence
|
|
No Author Comments
|
|
|
Acknowledgement
|
|
No Acknowledgement
|
|
|
Other References
|
|
No Other References
|
|
|
|
|