Introduction
Despite publication of the Benzodiazepine Committee’s report1 and good practice guidelines2 in 2002, there is evidence that inappropriate benzodiazepine prescribing (i.e. prescribing for durations in excess of 2-4 weeks) persists in Ireland in both general practice3 and residential care settings4. There are also ongoing concerns over the extent to which benzodiazepines are implicated nationally in cases of problem drug use5 and deliberate self-harm6. The use of Z-drugs (e.g. zopiclone, zolpidem) has reportedly increased over time in Ireland1 and other European countries7. Any trend towards increased use of Z-drugs over benzodiazepines would not constitute a favourable development as there is no compelling evidence of a clinically useful difference between benzodiazepines and Z-drugs in terms of their effectiveness or potential for adverse effects, dependence or abuse8. The Benzodiazepine Committee stated that its recommendations applied equally to benzodiazepines and Z-drugs owing to similarities in the two drug classes’ pharmacology1. The lack of any distinct difference in clinical benefit between the two classes is further reflected by the addition in 2012 of Z-drugs to the Beers criteria, some of the most widely used criteria for assessing potentially inappropriate prescribing in older patients9.
The Benzodiazepine Committee recommended the introduction of a system for auditing benzodiazepine prescribing in Ireland1. It was intended that prescribing practices would be reviewed regularly and that appropriate support and advice would be provided to prescribers where required. However, it took almost a decade for this recommendation to be acted upon10. This study was conducted prior to implementation of the national auditing system. The aim of the study was to assess benzodiazepine and Z-drug prescribing practices in terms of the level of prescription compliance with key components of the relevant prescribing guidelines2 relating to prescribed dose, duration and patients’ age profile. Previous evaluations11,12 have focused on estimating general parameters such as prevalence of use from prescription claims data as opposed to assessing the quality of individual prescriptions. As amendments to the existing Misuse of Drugs Regulations are currently being considered by government13 which, if passed into law, will have significant implications for the prescribing of benzodiazepines and Z-drugs in Ireland, it is timely to report the results of this assessment.
Methods
An audit of benzodiazepine and Z-drug prescriptions was conducted prospectively by a convenience sample of community pharmacists over a four week period in 2011. Pharmacists were recruited using a randomised and geographically stratified quota sampling method. A total of 290 pharmacists across the Republic of Ireland agreed to receive an audit booklet with a view to possible participation. The study was entirely anonymous. Pharmacists were issued a booklet in which they could evaluate up to 120 prescriptions using a questionnaire-styled system with Yes/No response options. Evaluation was based on prescription compliance with key guideline recommendations2 relating to prescribed dose, patient age and treatment duration (see Table 1). The audit questions were designed to assess prescribing practices while simultaneously being quick and easy to complete. No distinctions were made between health schemes. It was intended that all benzodiazepine and Z-drug prescriptions would be evaluated prospectively by participating pharmacists as they were presented in the pharmacy over a continuous four week period. Data validation and analysis were undertaken using SPSS v.18 (SPSS Inc., Chicago, IL, USA). Ethical approval was granted by the Faculty of Health Sciences Research Ethics Committee, Trinity College Dublin.
Results
Response rate
81 booklets with usable data were returned, yielding a response rate of 28%. Data were received from at least one pharmacy in each county in the Republic of Ireland with the exception of counties Clare and Westmeath. A further 7 booklets were returned that did not specify the county in which they were completed.
General analysis
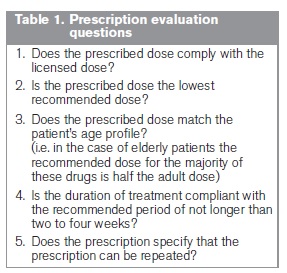
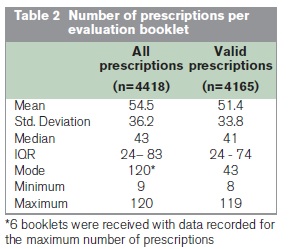
Data were collected on a combined total of 4,418 prescriptions. Following validation of the dataset, 253 (5.7%) prescriptions were either incompletely assessed (n=28) or deemed to have at least one conflicting response (n=225), such as stating that the dose was both unlicensed and the lowest recommended. These prescriptions were excluded from detailed analysis, yielding 4,165 valid prescription records. Summary statistics of the number of prescriptions per evaluation booklet are shown in Table 2. Excluding the small number of invalid records had no significant impact on the overall results. Analysis of the 4,165 valid records is presented in Figure 1.
The compliant prescription
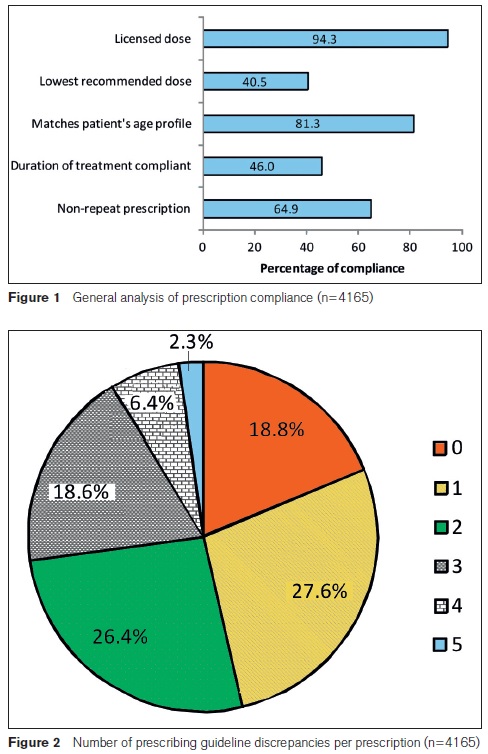
Figure 2 shows that fewer than one fifth (18.8%) of prescriptions complied with all the assessment criteria. The majority of prescriptions (53.7%) had at least two discrepancies and more than a quarter (27.3%) had three or more discrepancies.
Discussion
The results indicate that benzodiazepines and Z-drugs continue to be widely and frequently prescribed in Ireland, as data were collected on a large volume of prescriptions over a relatively short period. If prescriptions were assessed over the intended four week timeframe, and taking the average number of prescriptions evaluated (n = 54.5) across the 81 participating pharmacies, this would yield an average of approximately two prescriptions presented in each pharmacy daily. This is consistent with the findings of our previous research14, as well as annual reports of national reimbursement claims data published by the Primary Care Reimbursement Service which have repeatedly shown that benzodiazepines and Z-drugs feature among the most commonly dispensed medications on both public and private health schemes in Ireland15. The findings also indicate that there is not uniform adherence to the recommendations and prescribing guidelines published by the Benzodiazepine Committee more than a decade ago. For example, fewer than one in five prescriptions (18.8%) complied with all the key guideline criteria2 and the majority of prescriptions (53.7%) had at least two discrepancies. The most commonly identified problems were that the lowest recommended dose was not prescribed (59.5% of cases) and the duration of treatment was non-compliant with the recommended period (54.1% of cases). These findings are consistent with previous related research11,12,16. For example, the percentage of prescriptions that were non-compliant in terms of treatment duration (54.1%) closely mirrors the findings of Henman et al.11 who reported that in 2002 almost half (48.9%) of General Medical Services (GMS) patients in the former North Eastern Health Board region receiving benzodiazepines or Z-drugs were obtaining them for extended periods (i.e. longer than 3 months). While there may have been cases where prescribers were justified in deviating from guideline recommendations for reasons not immediately apparent to participating pharmacists, it is unlikely that such cases could have accounted for the high level of discrepancies identified in the current study. Overall, the findings suggest that little progress has been made in improving the prescribing of these medications in Ireland since publication of the Benzodiazepine Committee’s report.
The interim National Drugs Strategy17 noted that implementation of the Benzodiazepine Committee’s recommendations had been slow and among the issues relating to the Committee’s report needing to be addressed were the monitoring of prescribing practices, inappropriate use/supply and guideline implementation. Subsequent to this study there have been a number of important developments with direct implications for the prescribing of benzodiazepines and Z-drugs in Ireland. An auditing system of prescribing practices has been implemented nationally10 and self-auditing has been endorsed by the Irish College of General Practitioners, with guidance available to GPs on completing the various stages of a benzodiazepine prescribing audit cycle18. In addition, amendments to the existing Misuse of Drugs Regulations are currently being considered by government13. However, these measures alone are unlikely to be sufficient in effecting the changes necessary to improve benzodiazepine and Z-drug prescribing practices. Audit coupled with feedback can be effective in improving professional practice but the effects are generally small to moderate19. Furthermore, experience from implementing additional legislative measures has found that some degree of reduction in benzodiazepine use can be achieved but with the potential for unintended consequences, such as benzodiazepines being replaced with less favourable drug alternatives20.
The current study has a number of strengths. Firstly, participation was entirely voluntary and no form of incentive was offered. This supports the findings as an objective and unbiased assessment of benzodiazepine and Z-drug prescribing practices in Ireland. The relatively low proportion (5%) of overall prescriptions that had conflicting responses indicates that participating pharmacists were both capable and reliable in carrying out the assessment correctly and consistently. Owing to the sampling method employed and the 28% response rate, the possibility of response bias cannot be excluded. However, the participation rate was comparable to or greater than that in other benzodiazepine prescribing evaluations21-23 which relied on voluntary GP participation. A high volume of prescription data was obtained from a wide geographical spread which strengthens the findings as an assessment of prescribing practices on a national scale. In designing the evaluation questions, it was felt that it that would be more practical to have one set of assessment questions with relatively universal applicability to all prescription types, rather than making distinctions based on individual health schemes. Based on the volume of prescriptions on which data were collected, as well as informal feedback, it would appear that this held true in practice. However, this carried an inherent limitation for the study insofar as the assessments made relating to duration of treatment and repeat prescribing were curtailed by the health scheme set-up in Ireland and are likely to represent an underestimate. This is because GMS prescriptions are typically issued for a maximum duration of four weeks. Despite the existence of specific repeat GMS prescription forms, it is not uncommon for repeat supplies to be issued on identical single prescription forms that have been forward dated. This means that single GMS prescriptions, when assessed on an individual basis, could appear to comply with the recommended duration. However, without actually specifying repeat supply, post-dated GMS prescriptions could effectively facilitate it. Thus the actual proportion of prescriptions that were non-compliant in terms of treatment duration and repeat supply is likely to be higher than reported.
As was acknowledged by the Benzodiazepine Committee1, any single initiative is limited in the extent of the contribution it can make to address the problems associated with the use of drugs such as benzodiazepines and Z-drugs. However, evaluations of prescribing can provide valuable insights which are important in the process of changing prescribing practices24. As already noted, the use of community pharmacists for this study meant prescriptions were reviewed in the absence of information from prescriber-patient consultations that might have influenced prescribing decisions, hence the factors underlying deviations from guidelines could not be explored. However, the detected frequency of extended duration and repeat prescriptions, in particular, reveals the potential scope for other forms of intervention such as brief interventions25, which have shown considerable promise in reducing long-term benzodiazepine use. The study also shows the importance of, and necessity for, ongoing national monitoring and auditing of prescribing practices for benzodiazepines and Z-drugs. Based on the findings, interventions involving patients, prescribers and pharmacists should be pursued to improve the prescribing and use of benzodiazepines and Z-drugs in Ireland.
Correspondence: S Ryder
School of Pharmacy and Pharmaceutical Sciences, Trinity College, Dublin 2
Email: [email protected]
Acknowledgements
The researchers are grateful to all of the community pharmacists who participated in this study.
Funding
C Cadogan was supported in undertaking this research by an Ussher Fellowship awarded by TCD.
References
1. Ireland. Benzodiazepine Committee, Report of the Benzodiazepine Committee 2002, Dublin: Department of Health and Children.
2. Ireland. Department of Health and Children, Benzodiazepines: Good practice guidelines for clinicians. 2002, Dublin: Department of Health and Children.
3. Ryan C, O'Mahony D, Kennedy J, Weedle P, Byrne S. Potentially inappropriate prescribing in an Irish elderly population in primary care. Br J Clin Pharmacol, 2009. 68: 936-47.
4. Ryan C, O'Mahony D, Kennedy J, Weedle P, Cottrell E, Heffernan M, O'Mahony B, Byrne S. Potentially inappropriate prescribing in older residents in Irish nursing homes. Age Ageing, 2013. 42: 116-20.
5. Bellerose D, Lyons S, Carew AM, Walsh S, Long J. Problem benzodiazepine use in Ireland: treatment (2003 to 2008) and deaths (1998 to 2007). HRB Trends Series 9, 2010, Health Research Board: Dublin.
6. National Suicide Research Foundation, National Registry of Deliberate Self Harm Annual Report 2011, 2012, National Suicide Research Foundation: Cork.
7. Reed K, Bond A, Witton J, Cornish R, Hickman M, Strang J. The changing use of prescribed benzodiazepines and Z-drugs and of over-the-counter codeine-containing products in England: a structured review of published English and international evidence and available data to inform consideration of the extent of dependence and harm, 2011 The National Addiction Centre, King’s College London and the School of Social and Community Medicine, University of Bristol.
8. National Institute for Health and Clinical Excellence, Guidance on the use of zaleplon, zolpidem and zopiclone for the short-term management of insomnia. 2004.
9. American Geriatrics Society 2012 Beers Criteria Update Expert Panel, American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc, 2012. 60: 616-31.
10. Shannon, J. HSE benzodiazepine letters to encourage quality prescribing. 2012; Available from: http://www.medicalindependent.ie/page.aspx?title=hse_benzodiazepine_letters_to_encourage_quality_prescribing.
11. Henman M, Vivero L, Gustafsson A, Mulvenna K. Benzodiazepine usage in the North Eastern Health Board region of the Republic of Ireland, 2004, Trinity College Dublin.
12. Flynn, K., Minor tranquillisers and sedatives: Use and misuse in the West of Ireland, 2009, Western Region Drugs Task Force: Galway, Ireland.
13. Department of Health (Ireland), Consultation draft: Misuse of Drugs (Amendment) Regulations, 2013 to amend the Misuse of Drugs Regulations, 1988 as amended, 2013.
14. Cadogan CA, Ryder SA. Community pharmacists' views on benzodiazepine prescribing and supply. Int J Clin Pharm, 2012. 34: 238.
15. Primary Care Reimbursement Service. Annual reports of statistical analysis of claims and payments (2005-2011). Available from: http://www.hse.ie/eng/staff/PCRS/PCRS_Publications/.
16. Cahir C, Fahey T, Teeling M, Teljeur C, Feely J, Bennett K. Potentially inappropriate prescribing and cost outcomes for older people: a national population study. Br J Clin Pharmacol, 2010. 69: 543-52.
17. Ireland. Department of Community Rural and Gaeltacht Affairs, National Drugs Strategy (interim) 2009-2016, 2009, Department of Community, Rural and Gaeltacht Affairs: Dublin.
18. Irish College of General Practitioners. Benzodiazepine prescribing: Sample audit. 2012; Available from: http://www.icgp.ie/speck/properties/asset/asset.cfm?type=Document&id=E9A952A6-19B9-E185-8327C068C400C284&property=document&filename=BENZODIAZEPINE_PRESCRIBING_060613.pdf&revision=tip&mimetype=application%2Fpdf&app=icgp&disposition=attachment.
19. Ivers N, Jamtvedt G, Flottorp S, Young JM, Odgaard-Jensen J, French SD, O'Brien MA, Johansen M, Grimshaw J, Oxman AD. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev, 2012. 6: p. CD000259.
20. Fisher J, Sanyal C, Frail D, Sketris I. The intended and unintended consequences of benzodiazepine monitoring programmes: a review of the literature. J Clin Pharm Ther, 2012. 37: 7-21.
21. Hughes IM, Holden JD, Tree AM. Audit as a method of reducing benzodiazepine prescribing in general practice. Journal of Clinical Effectiveness, 1997. 2: 79-82.
22. Pimlott NJ, Hux JE, Wilson LM, Kahan M, Li C, Rosser WW. Educating physicians to reduce benzodiazepine use by elderly patients: a randomized controlled trial. CMAJ, 2003. 168: 835-9.
23. Baker R, Farooqi A, Tait C, Walsh S. Randomised controlled trial of reminders to enhance the impact of audit in general practice on management of patients who use benzodiazepines. Qual Health Care, 1997. 6: 14-8.
24. de Vries CS, Tromp TF, Blijleven W, de Jong-van den Berg LT. Prescription data as a tool in pharmacotherapy audit (I). General considerations. Pharm World Sci, 1999. 21: 80-4.
25. Mugunthan K, McGuire T, Glasziou P. Minimal interventions to decrease long-term use of benzodiazepines in primary care: a systematic review and meta-analysis. Br J Gen Pract, 2011. 61: e573-8.