Introduction
As of September 2012 there is new guidance for the management of paracetamol poisoning in the UK1 following recommendations made by the Commission for Human Medicines2. Ireland has since adopted the same guidelines from January 2013. Paracetamol is metabolised in the liver to a toxic metabolite p-benzoquinone imine. This is then conjugated with glutathione and inactivated. In paracetamol overdose there is rapid depletion of glutathione stores and hence accumulation of the toxic metabolite. Symptoms of paracetamol poisoning are highly dependent on time of presentation following the overdose. Vomiting due to the drug occurs in less than 12 hours, followed by vomiting due to liver damage in 12-36 hours. From 24-72 hours patients can develop right upper quadrant pain and hypoglycaemia. After 72 hours patients typically present with cerebral oedema and/or encephalopathy. Risk factors leading to accelerated glutathione depletion include malnutrition, chronic alcohol abuse and enzyme-inducing drugs. Intravenous N-acetylcysteine is the treatment of paracetamol overdose and is virtually 100% effective in preventing liver damage when given within 8 hours of the overdose. After this time efficacy falls substantially.
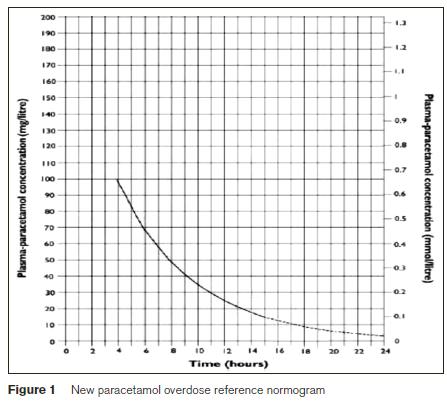
In the new guidelines all patients are now treated based on a single treatment line (previously the high risk line) on the treatment normogram, Figure 1. The loading dose of N-acetylcysteine is now given over 60 minutes to minimise the risk of common dose-related adverse reactions. The toxic dose of paracetamol has been reduced to 75mg/kg from 150mg/kg. The aim of this study was to determine to what extent the introduction of the new UK guidelines1 will result in a greater number of patients needing treatment with N-acetylcysteine.
Methods
We performed a retrospective review on all patients who had paracetamol levels done in the ED between September 2011 and August 2012 in University Hospital Galway. Data was collected from the biochemistry laboratory computer system and ED notes reviewed through the ED information system. Demographics collected were age of patient at time of overdose, gender, time of overdose and time of blood sample collection, and whether N-acetylcysteine was used. Risk assessment documentation was also reviewed. All positive paracetamol results were plotted on the new UK guideline normogram.
Results
A total of 523 patients were identified as having had paracetamol levels done in 12-month study period. Of these 95 (18%) had detectable paracetamol levels. Fourteen ED notes were not available for review and therefore were eliminated from the study. Thirteen (13%) charts had no documented time of ingestion or time paracetamol level was taken. Of the 13 with no documentation, only 6 (6%) had presented following an overdose. The remainder (7) had a toxicology screen as part of their work up. A total of 74 patients were therefore evaluated. Most of these patients presented between 8pm and 8am (63%), with a male to female ratio of 1:1.7. The average age was 29 years with a range of 14 – 69 years (figure 2). Eighteen (24%) patients were treated with N-acetylcysteine in accordance with the then current guidelines. Assessing these patients against the new UK guidelines would have resulted in treatment and therefore admission of 21 (28%) patients over the 12-month period. Risk assessment was only documented in 27 (36%) patients.
Discussion
This study confirms that most patients who present to ED following paracetamol overdose do not require treatment with N-acetylcysteine. It also suggests that the introduction of the new UK guidelines1, which will lower the treatment threshold for all patients to the previous high risk line is likely to result in only a small increase in the number of patients requiring treatment. The need for risk assessment, which was poorly recorded in this study will also be eliminated. The new guidelines will simplify the assessment and management of paracetamol overdose without causing a large increase in the number of patients requiring admission for treatment.
Correspondence: G Nfila
Department of Emergency Medicine, University College Hospital, Newcastle Rd, Galway
Email: [email protected]
Acknowledgements
D Griffin, Department of Biochemistry, University Hospital Galway, Galway
References
1. Toxbase.org; accessed on 30/01/2013
2. Medicines and Healthcare Products Regulatory Agency; mhra.gov.uk; accessed on 30/01/2013