Introduction
Acute gastroenteritis is a leading cause of morbidity and mortality, and is a common presentation to Emergency Departments (EDs). Our Emergency Department currently sees 32,000 children per year, with over two thousand visits due to gastroenteritis. The mainstay of treatment is to maintain hydration during this acute illness. Oral Rehydration Therapy (ORT) is well established as the preferred treatment modality and is endorsed by the World Health Organisation1 and other major health organisations2,3. Ongoing vomiting from gastroenteritis is uncomfortable for children and distressing to families. Vomiting during the rehydration process can result in oral attempts being bypassed for intravenous rehydration. Measures to control vomiting increase success rates of oral rehydration.
Traditional anti-emetics have been unsuccessful in children as they are associated with significant adverse effects and commonly cause drowsiness which can limit a child’s ability to drink fluids. Ondansetron is the only non-sedating anti-emetic with consistent, proven efficacy in reducing vomiting from gastroenteritis4. Ondansetron, a selective serotonergic 5-HT3 receptor antagonist has potent antiemetic properties. It has been shown in numerous well designed studies in children to reduce episodes of vomiting, reduce the need for IVF and improve oral intake 5-8. It decreases the episodes of vomiting when given with ORT6,8 and has become an established adjunct for ORT. We describe the addition of oral Ondansetron to facilitate our Emergency Department’s already established successful protocol of oral rehydration in the Waiting Room9 and detail a retrospective observational study to evaluate whether this intervention would reduce the need for intravenous fluids and hospital admission in our ED.
Methods
Children up to sixteen years and weighing more than 5 kilograms who presented to the Emergency Department (ED) with symptoms suggestive of acute gastroenteritis and poor oral intake were initially assessed by a triage nurse. Our operational definition of gastroenteritis was and an acute diarrhoeal illness with or without vomiting. Those with a presumptive diagnosis of acute gastroenteritis, who did not have signs or symptoms suggestive of other illnesses, were started on a trial of oral rehydration after triage and prior to medical assessment.
Children were excluded if they were suffering from severe dehydration, change in consciousness or severe abdominal pain. Parents were provided with an information sheet, a syringe and a cup of electrolyte solution. Children who failed this initial trial (vomited or refusing fluids) traditionally received intravenous fluids following a medical assessment. On introduction of this new protocol those who failed the initial trial of ORT received a single weight adjusted dose of Ondansetron. The weight based dose was 1mg for children weighing 5-10kg, 2mg for 10-15kg, 4mg for 15-30kg and 8 mg for children weighing 30 kg or more. The Ondansetron was administered in an oral lysophilisate form (“Zofran Melts”) which when placed on the child’s tongue dissolves and child is asked to swallow 5 seconds later. If vomiting occurred within thirty minutes the dose was repeated. Parents were advised to recommence a trial of oral fluids after 30 minutes. If, at that stage, the child remained unable to tolerate oral fluids or refused to drink, we reverted to usual ED care and administered intravenous fluids following medical assessment. Patients were either discharged when they demonstrated adequate oral intake or admitted if they required ongoing intravenous therapy.
Following a two week introductory period in October 2009 involving nursing and medical staff education, data was collected over a six week period (October to December 2009). All patients with a discharge diagnosis of gastroenteritis on the Symphony Emergency Department electronic database were included in the study. Data collected included age, sex, weight, length of ED stay and documentation of ORT. Outcome measures investigated included number of children requiring intravenous fluids, admission and representation rates. Results were compared to a similar six week period in 2008 when children received trials of oral rehydration therapy without an anti-emetic.
Children were excluded from the study if clinical notes documented an alternate diagnosis or notes were incomplete. Children presenting with hypoglycaemia secondary to gastroenteritis were also excluded as these patients traditionally received intravenous fluids in our Emergency Department. Children were counted as a representation if they returned to the ED within seven days with ongoing symptoms. The representation data was excluded from analysis of baseline patient characteristics but was included in study endpoint analysis. Results were tested for significance using the Chi- squared test. The work entailed a retrospective review of electronic patient records and ethics committee/IRB approval is not required for such a study at our institution.
Results
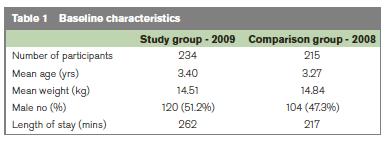
During the 2009 study period there were 245 attendances with a discharge diagnosis of gastroenteritis, with 246 attendances in the 2008 comparison group. Forty two patients were excluded from the groups (15 patients with hypoglycaemia, 16 with a diagnosis other than gastroenteritis and 11 patients had incomplete clinical notes). There were 234 patient notes available for further analysis in the study group, and 215 in the comparison group. Baseline characteristics were comparable with respect to patient weight, sex and age (Table 1). There was a 19% reduction in the number of children receiving intravenous fluids following the addition of Ondansetron to our ORT programme (88/215 patients in 2008, 51/234 in 2009, p < 0.0001, Table 2). Admission rates reduced slightly from 31/215 in 2008 to 30/234 in 2009, but this result was not statistically significant (p=0.62).
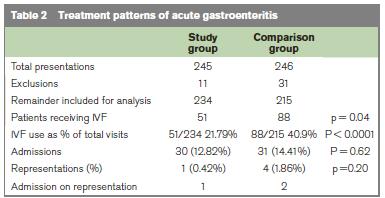
Thirty four patients received Ondansetron during the six week study period, twenty nine had vomited while attempting ORT and the remainder had refused fluids. The average age of children in this group was 3.27 years with no significant gender differences (18 males, 16 females). Twenty seven children (79%) subsequently completed a successful trial of ORT. All of the remaining seven children received intravenous fluids, three ultimately succeeded with oral hydration and were discharged from the Emergency Department. The remaining four children were hospitalised. Overall representation rates were low - a single representation in the 2009 group (subsequently discharged) and four representations in the comparison group, two of whom were admitted. Only one child who had received Ondansetron represented the following day, due to ongoing symptoms. The child was tolerating fluids well and was discharged home following reassurance.
Discussion
The addition of a single dose of Ondansetron to our already established oral rehydration protocol for children in the waiting room with acute gastroenteritis and reduced oral intake has improved the success of this protocol. The intervention was well tolerated and we demonstrated a significant reduction in the number of children receiving intravenous fluids when compared to a group receiving ORT without an anti-emetic. This reduction, when extrapolated over a one year period of gastroenteritis presentations would result in approximately 380 less children receiving IVF in our Emergency Department each year. A more modest reduction (2%) in admission rates were observed over the six week period study period. Anecdotally as our protocol becomes more established we see a more significant impact on admission rates, mirroring other studies.
We acknowledge several limitations to our study. As a retrospective review it is difficult to control for other variables which may have influenced our positive results. Ondansetron has been a major role player in the reduction in IVF administration, but in this limited analysis we cannot exclude other confounding factors. We noted baseline characteristics to be similar between groups, and feel staffing levels and threshold for treatment interventions have not significantly differed over these two consecutive years. Total numbers of acute gastroenteritis presentations were also similar over both time periods. We did not undertake a formal cost benefit analysis or cost effectiveness analysis. However given the costs associated with intravenous treatments and hospital admission and the relatively low numbers needed to treat (NNT of 54) demonstrated in the literature, Ondansetron use in the ED is likely to be cost effective.
There are many barriers to both Oral Rehydration Therapy and anti-emetic use in children. ORT has been perceived to be time consuming and perhaps doesn’t meet parental expectations of an Emergency Department visit10 yet ORT is quicker to initiate and obviates the need for painful and sometimes difficult cannulation10. A previous publication from our ED demonstrated that children undergo hours of successful treatment while waiting to be seen, and that waiting room ORT became more effective during the times when the ED was busiest9. Despite convincing evidence of Ondansetron’s effects on oral hydration tolerance, there have been legitimate concerns that children would be inappropriately treated for the symptom of vomiting prior to making a clear diagnosis and mask a serious underlying illness. A large well designed retrospective review11 involving 19,857 children receiving Ondansetron in cases of suspected gastroenteritis in ED settings concluded its use did not appear to be associated with increased risks of masking serious diagnosis. We acknowledge that triage is not a diagnostic process and were reassured by the absence of any serious alternative diagnoses in those receiving Ondansetron in our study. As conclusions from this study are limited by a small sample size we plan to analyse data from a larger cohort of children who received Ondansetron in our ED to further validate our findings.
We found that the addition of oral Ondansetron to facilitate oral rehydration therapy in our Emergency Department a useful adjunct in managing children with dehydration secondary to acute gastroenteritis. It has reduced the rate of intravenous fluid use and hospital admission without significantly prolonging patient stays in the Emergency Department.
Correspondence: C Mullarkey
Emergency Department, National Children's Hospital, Tallaght, Dublin 24
Email: [email protected]
References
1. Victoria CG, Bryce J, Fontaine O, Monash R. Reducing deaths from diarrhoea through oral rehydration therapy. Bull World Health Organ 2000; 78: 1246-1255.
2. King CK, Glass R, Bresee JS, Duggan C. Managing acute gastroenteritis among children: oral rehydration, maintenance and nutritional therapy. MMWR Recomm Rep. 2003; 52:1-16.
3. American Academy of Pediatrics, Provisional Committee on Quality Improvement, Subcommittee on Acute Gastroenteritis. Practice Parameter: the management of acute gastroenteritis in young children. Pediatrics. 1996; 97:424-436.
4. DeCamp LR, Byerley JS, Doshi N, Steiner MJ. Use of antiemetic agents in acute gastroenteritis – a systematic review and meta-analysis. Arch Pediatr Adolesc Med. 2008; 162: 858 -865.
5. Freedman SB, Adler M, Seshadri R, Powell EC. Oral ondansetron for gastroenteritis in a pediatric emergency department. N Engl J Med 2006;354:1698-1705.
6. Ramsook C, Sahagun-Carreon I, Kozinetz CA, Moro-Sutherland D. A randomized clinical trial comparing oral ondansetron with placebo in children with vomiting from acute gastroenteritis. Ann Emerg Med 2002;39:397-403.
7. Roslund G, Heppes TS, McQuillen KK. The role of oral ondansetron in children with vomiting as a result of acute gastritis/gastroenteritis who have failed oral rehydration therapy: a randomized controlled trial. Ann Emerg Med 2008;52:22-29.
8. Cubeddu LX, Trujillo LM, Talmaciu I, Gonzalez V, Guariquata J, Seijas J, Millar IA, Paska W.Anti-emetic activity of ondansetron in acute gastroenteritis. Ailment Pharmacol Ther. 1997; 11:185-191.
9. Craven JA, Campbell L, Martin CT. Waiting Room oral rehydration in the Paediatric Emergency Department. Ir Med J. 2009 Mar; 102:85-7.
10. Spandorfer PR, Evaline AA, Joffe MD, Localio R, Shaw KN. Oral versus intravenous rehydration of moderately dehydrated children: a randomised controlled trial. Pediatrics 2005; 115: 295-301.
11. Sturm JJ, Hirsh DA, Schweickert A, Massey R, Simon HK. Ondansetron use in the Pediatric Emergency Department and effects on hospitalisation and return rates: are we masking alternate diagnoses? Ann Emerg Med. 2010; 55: 415-422.