M Callaghan, Y Doyle, B O’Hare, M Healy, L Nölke
Our Lady’s Children’s Hospital, Crumlin, Dublin 12
Abstract
Extra corporeal membrane oxygenation (ECMO) is a form of life support, which facilitates gas exchange outside the body via an oxygenator and a centrifugal pumping system. A paediatric cardiac ECMO programme was established in 2005 at Our Lady’s Children’s Hospital, Crumlin (OLCHC) and to date 75 patients have received ECMO, the majority being post operative cardiac patients. The outcome data compares favourably with international figures. ECMO has been most successful in the treatment of newborn infants with life threatening respiratory failure from conditions such as meconium aspiration, respiratory distress syndrome and respiratory infections. There is no formal paediatric respiratory ECMO programme at OLCHC, or anywhere else in Ireland. Currently, neonates requiring respiratory ECMO are transferred to centres in Sweden or the UK at an average cost of €133,000/infant, funded by the Health Service Executive E112 treatment abroad scheme. There is considerable morbidity associated with the transfer of critically ill infants, as well as significant psycho-social impact on families. OLCHC is not funded to provide respiratory ECMO, although the equipment and expertise required are similar to cardiac ECMO and are currently in place. The average cost of an ECMO run at OLCHC is €65,000. There is now a strong argument for a fully funded single national cardiac and respiratory paediatric ECMO centre, similar to that for adult patients.
Introduction
Extra corporeal membrane oxygenation (ECMO) is a form of extra corporeal life support (ECLS) that provides respiratory support by facilitating gas exchange outside the body via an oxygenator, as well as cardiovascular support via a centrifugal pumping system. Technological advancements in the last decade have seen ECMO become more sophisticated and widespread. An adult ECLS programme was established in the Mater Misericordiae University Hospital in 2009, and provides ECMO for adults with both cardiac and respiratory disease. A cohort of patients was treated with ECMO there during the H1N1 influenza pandemics1. Prior to this programme the majority of these patients would have either not survived or been transferred overseas for ECMO.
Our Lady’s Children’s Hospital cardiac ECMO programme
A paediatric ECMO programme was established in Ireland in 2005, for post-operative cardiac patients in Our Lady’s Children’s Hospital, Crumlin (OLCHC). As of 31st Decemeber 2011, 75 patients have received ECMO in OLCHC. The majority (63) was post-operative cardiac patients (mainly patients with complex congenital heart disease who could not be weaned from cardio-pulmonary bypass immediately post-operatively), five were cardiology patients and seven were respiratory patients. ECMO is provided in the eight bed cardiac intensive care unit; two nurses are required per patient on ECMO, meaning the overall unit capacity is reduced to seven beds when there is a patient on ECMO. There is no formal provision for paediatric patients requiring respiratory ECMO at OLCHC or anywhere else in Ireland.
Paediatric respiratory ECMO
Although ECMO was first developed for use in adults, it has been most successful in the treatment of newborn infants with life-threatening pulmonary failure2. Robert Bartlett, a thoracic surgeon, first described its use in 1974 in a neonate with severe meconium aspiration syndrome in his paper Esperanza3 (named after the baby). ECMO is beneficial in conditions such as meconium aspiration syndrome, primary pulmonary hypertension, congenital diaphragmatic hernia, respiratory distress syndrome and respiratory sepsis4. ECMO allows time for the lungs to recover, while maintaining adequate gas exchange. The UK Collaborative ECMO trial has shown it to be cost effective and reduce neurological morbidity and overall mortality5. The Extracorporeal Life Support Organization (ELSO) maintains a registry of cases in which ECMO was performed. Over 200 centres from around the world have contributed data. Currently, there are over 46,000 cases in the registry including over 29,500 newborns, 11,000 children, and 4,500 adults with respiratory and cardiac failure. The ELSO registry reports overall survival rates of 75% in neonatal respiratory failure6.
The current situation in Ireland is that children requiring ECMO for respiratory support are referred to centres in Sweden or the UK. These centres provide a retrieval service and generally patients are placed on ECMO by the retrieving team prior to air transfer. The cost is borne directly by the HSE E112 treatment abroad scheme, rather than by the referring hospital. A recent study by EL-Khuffash of 11 neonates requiring respiratory ECMO reports a total cost of €1,197,000 and a median total cost of €133,000 per infant transferred8. The cost of air transfer is €25,000. The cost of ECMO provision at one centre (Karolinska, Sweden) is €9,000 /day. In the event that a respiratory ECMO bed is not available abroad an economic and ethical dilemma ensues. As outlined ECMO for cardiac support is available at OLCHC; the lack of financial or resource provision for respiratory ECMO means it is not officially provided, although the equipment and expertise required to provide it are almost identical to that of cardiac ECMO. It seems unethical not to offer such support when it is clinically indicated, given that the equipment and skilled personnel are already available. Inevitably however, this will have an impact on the hospital budget, and presumably will affect the provision of other services within the hospital. The ethical concepts of beneficence and justice would appear to be in direct conflict. There are a number of issues pertaining to the provision of respiratory ECMO for children in Ireland, which are highlighted by two recent cases.
Case 1
A male infant with a congenital diaphragmatic hernia (CDH) and refractory hypoxia was referred to Sweden and the UK for ECMO; an iatrogenic lung contusion during chest drain insertion had compounded the hypoxia. No ECMO bed was available overseas but the consensus from these centres was that ECMO was clinically indicated, particularly given the reversibility of the lung contusion. In view of this, and in spite of the lack of a formal programme, ECMO was commenced at OLCHC. The infant was weaned from ECMO five days later and underwent surgical repair of his CDH.
Case 2
A female infant with acute respiratory distress syndrome (ARDS) secondary to adenovirus infection was referred to Sweden for ECMO. Severe refractory hypoxia necessitated urgent institution of ECMO and stabilisation in OLCHC by the local cardiac surgeons. She was then transferred to Karolinska, Sweden by their retrieval team for ongoing ECMO support.
In case 1, the lack of an ECMO bed overseas meant that unless ECMO was provided at OLCHC, the infant would almost certainly die. In view of this, and following consultation with hospital management, ECMO was commenced. During the five days of ECMO support the cardiac ICU was reduced from a capacity of eight to seven beds. The cost of equipment and staffing was met from the hospital budget. In case 2 urgency necessitated that the infant be cannulated and placed on ECMO in OLCHC, whilst awaiting arrival of the transfer team. The baby was then transferred by air to Sweden for ongoing ECMO support, a support that could have been provided at OLCHC at a lower cost. There is significant morbidity associated with the transfer of critically ill neonates. For example, a UK study on neonatal transfer found that of 2402 transfers over a seven year period, 562 were associated with at least one critical incident and that eight of these were potentially catastrophic incidents9. The psychological, social and economic burden that overseas transfer places on parents, at an already stressful time, must also be considered.
Time for a single paediatric cardiac and respiratory ECMO centre?
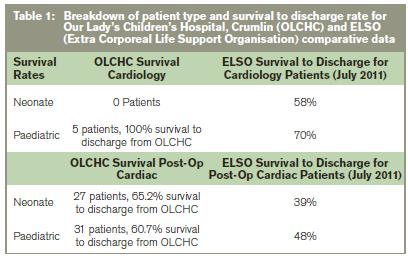
This discussion is not intended to debate the merits or otherwise of paediatric respiratory ECMO; that is a separate issue. It is intended to highlight the current inequity whereby high quality ECMO is available to cardiac patients (although the programme is only partially HSE funded), yet children with respiratory disease, in whom clinicians believe ECMO is indicated, are referred overseas. There is now a strong argument for a fully funded single national cardiac and respiratory paediatric ECMO programme, similar to that provided for adult patients. There is a proven track record of cardiac ECMO at OLCHC; although overall numbers are small the outcome results are good, with a 65% survival to discharge rate in neonatal cardiac surgery ECMO patients (compared with a 39% ELSO average for cardiac neonates). Indeed the OLCHC programme has been recognised internationally with the ELSO Clinical Excellence Award in 2010 (one of only four European centres to achieve this award). We estimate the average cost of an ECMO run at OLCHC to be €65,000, which compares favourably with the figures from EL-Khuffash et al of €133,000 abroad. The advantage of having both cardiac and respiratory ECMO provided at one site would mean maintenance of a critical patient load and the associated expert personnel and equipment.
Correspondence: M Callaghan
Our Lady’s Children’s Hospital, Crumlin, Dublin 12
Email: [email protected]
Acknowledgements
The contribution of the nursing staff of the cardiac intensive care unit and the staff of the department of clinical perfusion at Our Lady's Children's Hospital, Crumlin.
References
1. Nicolay N, Callaghan MA, Domegan LM, Oza AN, Marsh BJ, Flanagan PC, Igoe DM, O'Donnell JM, O'Flanagan DM, O'Hora AP. Epidemiology, clinical characteristics and resource implications of pandemic (H1N1) 2009 in intensive care units in Ireland. Crit Care Resusc. 2010 Dec;12:255-61
2. Philip J. Wolfson The development and use of extracorporeal membrane oxygenation in neonates. Ann Thorac Surg 2003;76:S2224-S2229
3. Bartlett RH. Esperanza. Presidential address. Trans Am Soc Artif Intern Organs 1985;31:723-6
4. McNally H, Bennett CC, Elbourne D, Field DJ. United Kingdom collaborative randomized trial of neonatal extracorporeal membrane oxygenation: follow-up to age 7 years. Pediatrics 2006; 117: e845-e854.
5. Petrou S, Bischof M, Bennett C, Elbourne D, Field D, McNally H. Cost-effectiveness of neonatal extracorporeal membrane oxygenation based on 7-year results from the United Kingdom Collaborative ECMO Trial. Pediatrics 2006; 117: 1640-1649
6 Tiruvoipati R, Pandya H, Manktelow B, Smith J, Dodkins I, Elbourne D, Field D. Referral pattern of neonates with severe respiratory failure for extracorporeal membrane oxygenation. Arch Dis Child Fetal Neonatal Ed 2008 ; 93: F104-F107
7. http://www.citizensinformation.ie/en/health/hospital_services/hospital_treatment_abroad.html
8. A EL-Khuffash, E Kieran, K Palmer, E Molloy. Neonatal Respiratory Extracorporeal Membrane Oxygenation (ECMO) Referrals. Ir Med J. 2011 Mar;104:78-81
9. SJ Moss, ND Embleton, AC Fenton. Towards safer neonatal transfer: the importance of critical incident review. Arch Dis Child. 2005; 90:729–732.