|
|
|
|
|
|
|
|
john lambert,Valerie Jackson,S Coulter-Smith,Marian Brennan,M Geary,T.Barry Kelleher,M O''Reilly,K Grundy,Norma Sammon,M Cafferkey
|
|
|
|
Ir Med J. 2013 May;106(5):136-9
|
J Lambert, V Jackson, S Coulter-Smith, M Brennan, M Geary, TB Kelleher, M O'Reilly, K Grundy, N Sammon, M Cafferkey
Rotunda Hospital, Parnell St, Dublin 1
Abstract
The aims of this study were to pilot universal antenatal HCV screening and to determine the true seroprevalence of HCV infection in an unselected antenatal population. A risk assessment questionnaire for HCV infection was applied to all women booking for antenatal care over a 1-year period. In addition the prevalence of anti-HCV antibody positive serology in this population was determined. Over the course of the year, 9121 women booked for antenatal care at the Rotunda and 8976 women agreed to take part in the study, representing an uptake of 98.4%. 78 (0.9%) women were diagnosed as anti-HCV positive, the majority of whom were Irish (60.3%) or from Eastern Europe (24.4%). 73% of anti-HCV positive women reported one or more known risk factor with tattooing and a history of drug abuse the most commonly reported. 27% (n=21) of anti-HCV positive women had no identifiable risk factors. Due to selective screening, seroprevalence of HCV is impossible to accurately calculate. However the universal screening applied here and the high uptake of testing has allowed the prevalence of anti-HCV among our antenatal population to be calculated at 0.9%. A significant proportion (27%) of anti-HCV positive women in this study reported no epidemiological risk factors at the time of booking and so were identified only as a result of universal screening. This provides persuasive evidence for the inclusion of HCV testing with routine antenatal screening or at a minimum highlights the need for ongoing review of selective screening criteria.
Introduction
Hepatitis C virus (HCV) is a blood borne virus spread through contaminated blood or bodily fluids. First identified in 1989, it has since emerged as a leading cause of liver cancer and liver transplants in Europe and the USA. It is estimated that 3% of the world’s population is chronically infected with HCV and 3 to 4 million people are newly infected annually.1 Acute infections are often asymptomatic and so, many cases are undiagnosed. Up to 75% of acute infections progress to chronic infection and as such are at risk of progressive inflammatory liver disease, cirrhosis, hepatocellular carcinoma and liver related death.2 The incidence of HCV in Ireland in 2010 was reported as 29 cases per 100,000.3 Most infections are attributable to the sharing of needles and other drug paraphernalia, with reported prevalence rates of 66-69% among injecting drug users.4,5 Other modes of transmission include receipt of contaminated blood products (pre-1992), occupational exposures, heterosexual and vertical transmission. The antenatal prevalence of HCV infection in Europe ranges from <1% to 2.5%. With the introduction of routine screening of blood/blood products for HCV, vertical transmission is now the dominant mode of transmission in children. Transmission can occur either late in pregnancy or at the time of delivery and is largely dependent on maternal viraemia.6,7 Vertical transmission rates of between 5 and 15% have been reported.
Previous studies at the Rotunda revealed a vertical transmission rate of approximately 6.4%.8 However higher vertical transmission rates of up to 40% have been recorded in HIV co-infected mothers.
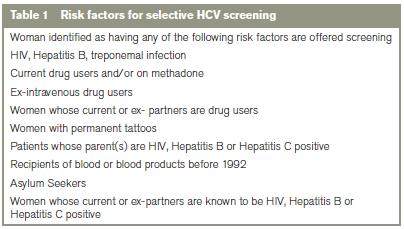
At the Rotunda Hospital, selective screening for HCV is carried out on perceived “high risk” women (Table 1) and as such true seroprevalence rates in the antenatal population aren’t known. Studies elsewhere have shown that selective screening fails to identify all HCV women – perhaps up to 50%.9 The aim of this study is to pilot universal antenatal screening for HCV and to determine the seroprevalence in our antenatal population and the associated risk factors for infection. This information will underpin future screening strategies for HCV in antenatal and other Irish populations.
Methods
From June 2007 to June 2008, all women booking for antenatal care were offered an anti-HCV antibody test as part of their routine antenatal screening. Each woman was provided with an information leaflet, consent form and a risk assessment questionnaire. Once signed consent was obtained, the booking midwife completed the risk assessment questionnaire and the anti-HCV antibody test was requested (Abbot AxSym Analyser v3.0). All positive samples were sent to the National Virus Reference Laboratory for confirmation, PCR genotyping and viral load determination. Women with positive serology were informed of results and counselled by the Infectious Disease Liaison Midwife. Each woman was referred to adult Infectious Disease/Hepatology services for further assessment and arrangements were made for paediatric follow-up. Univariate associations between categorical variables were explored using the chi2 or Fisher’s exact test. Multivariate analysis was performed using logistic regression. All tests were 2-tailed; p<0.05 was considered significant (analyses performed using SPSS v18).
Results
Over the course of the year 9121 women booked for antenatal care and 8976 women agreed to take part in the study, representing an uptake of 98.4%. Seventy-eight women in the cohort tested positive for anti-HCV antibodies giving a seroprevalence rate of 0.9%. RT-PCR analysis was carried out on 67/78 positive samples. Of these 43 (64%) were positive for HCV-RNA. Viral loads were available in 23/78 and 6 (26%) of these women had viral loads greater than 1x106 copies/mL. Genotype was available for 14/78 samples only. The most prevalent genotype was 1B (6/14) followed by 1A (4/14). Among the 78 anti-HCV positive women, one woman was also co-infected with hepatitis B, while two women were co-infected with HIV.
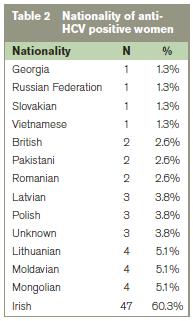
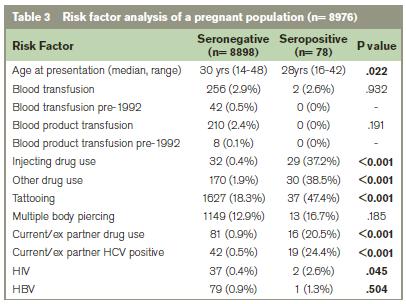
The majority of anti-HCV positive women were Irish (60.3%) (Table 2). Most women (72.8%) cited one or more risk factor for HCV infection. The most frequently disclosed risk factors were tattooing (47.4%), injecting drug use and other drug use (e.g. cocaine, cannabis) (Table 3). 27% of the anti-HCV positive women had no identifiable risk factor for infection. Over half (57%) of these women were from Eastern European countries. Univariate analysis revealed a significant association between several risk factors (incl. tattooing, injecting or other drug use, a current/ex partner infected with HCV or a current/ex partner with a history of IDU) and anti-HCV antibody status (p<0.001). In multiple regression only injecting drug use and tattooing remained significantly associated with anti-HCV status (p<0.001 and p<0.05 respectively).
Discussion
This large single centre study has enabled an accurate assessment of HCV antibody prevalence in the Irish antenatal population. The calculated seroprevalence rate of 0.9% is similar to that found in other studies and is close to the 1% seroprevalence threshold recommended for the introduction of universal screening.9,10 The high participation rate in the study (98.4%) also indicates that universal screening is acceptable to the majority of women. It is well known that maternal viraemia is associated with an increased risk of vertical transmission.11,12 Almost two thirds of women tested here were viraemic and 6/23 had a viral load greater than 106 copies/mL, putting them in the higher risk category for mother to child transmission.
The majority of HCV infections occurred in Irish women. Almost a quarter of infections were in women from Eastern European countries – a dramatic increase on the findings of an earlier review where <1% of HCV infections were represented by this group.8 This difference is likely a reflection of the changing demographics of Ireland in recent years rather than a change in HCV epidemiology. Of particular note however is that that almost two thirds of the Eastern European women had no identifiable risk factor for HCV infection. The high prevalence of HCV in Eastern Europe is well documented, with incidence rates of acute infection of between 2.3 and 9.0 per 100,000 population recorded in 1997.13 Available information on the risk factors for infection indicates nosocomial transmission plays a major role in these countries. Diagnostic or other treatment procedures in hospitals were cited as a source of infection in 59-65% of cases in Poland, 59% in Latvia and 46% in Lithuania.13 A study of 711 HCV positive patients conducted in Bucharest revealed parenteral procedures in hospitals accounted for 49.8 % of HCV infections, while surgery and blood transfusions account for 21.2% and 14.5% respectively.14
As expected, illegal drug use emerged as a significant risk factor for infection. However over a quarter of the HCV positive women in this study had no identifiable risk factor at the time of booking for antenatal care. In the absence of any self reported risks, it is possible that other risk factors such as heterosexual transmission need to be considered. To date the role of heterosexual transmission in the epidemiology of HCV remains controversial. One study calculated a heterosexual transmission rate of 5% in monogamous spouses of patients with chronic HCV infection and viraemia. However exclusion of spouses with differing genotypes and/or additional independent risk factors for infection, the interspousal transmission rate was only 1.25%.15 In contrast another study suggested heterosexual transmission was the contributing risk factor in 8% of HCV infections in an antenatal cohort.8 In this cohort 24% of HCV positive women revealed a current/ex partner was positive for HCV. In all but 1 case additional risk factors were disclosed, making conclusions about the role of sexual transmission difficult. HCV antenatal screening policies vary greatly across Europe; one review of 31 centres revealed an almost equal distribution of centres practicing universal screening, selective screening and no screening at all.16 However the epidemiology of infection across Europe is constantly changing due to immigration from endemic areas and so prevalence data needs to be constantly reviewed to estimate the current and future burden of HCV infection and ensure appropriate policies are in place.17,18
At present the Rotunda hospital like many other maternity hospitals, operates a selective screening policy based on disclosed risk factors; however the data presented here reveals the shortcomings of this policy. Universal screening is recommended in populations where the prevalence rate is ≥1%. Others have discussed the pros and cons of selective testing.19,20 Relying on self-reported risk behaviours and the underestimation of the importance of other risk factors such as heterosexual transmission and nosocomial transmission in high prevalence countries, means a significant proportion of cases will invariably remain undiagnosed under the current guidelines. This could have potential health implications for both mother and child.21 While pregnancy management for HCV positive women is no different than for non-infected women (except if HIV co-infected), reports on the risks of obstetrical complications due to maternal HCV status are varied. In one US study, infants born to HCV positive mothers had an increased risk of low birth weight, being small for gestational age and admission to the NICU.22 In contrast a smaller Irish study failed to show any increase in risk between infants born to HCV positive women versus a control group.23
Although there are currently no treatment options available in pregnancy to minimise the risk of vertical transmission, timely identification of infection will permit interventions to limit/reduce the risk of progression of liver disease e.g. avoidance of hepatotoxic medications and alcohol. Diagnosis also enables active immunisation against other types of infective hepatitides. In addition it has been suggested that the postpartum period is an optimal time to initiate treatment as the loss of pregnancy induced immunosuppression post delivery leads to decreased HCV RNA titres.24,25 Early referral of mothers to treatment programmes could potentially eradicate their HCV so that future unborn children are not at risk of this infection. The provision of antenatal care can represent a unique opportunity to test women who otherwise may not have sought testing. While the prevalence of HCV noted here is below the recommended threshold for implementation of universal screening, the high proportion of HCV positive women with no epidemiological risk factors is cause for concern. Although the possibility of non-disclosure of perceived “negative” or “unacceptable” behaviours cannot be excluded, the data presented here provides persuasive evidence for the inclusion of HCV testing with routine antenatal screening or at a minimum highlights the need for ongoing review of the selective screening approach.
Correspondence: JS Lambert
Catherine McAuley Research Centre, Mater Misericordiae University Hospital, Nelson St, Dublin 7
Email: [email protected]
Acknowledgements
The financial support of the Friends of Rotunda; the assistance of the midwifery and administration staff at the Rotunda Hospital and A Jackson for her contribution during the early stages of the research.
References
1. Hepatitis C, WHO factsheet. July 2012; Available at http://www.who.int/mediacentre/factsheets
2. Irving W L, Salmon D, Boucher C, Hoepelman I M. Acute Hepatitis C Virus Infection. Eurosurveillance. 2008; 13:Issues 4-6.
3. Epidemiology of Hepatitis C in Ireland. 2010; available at www.hpsc.ie
4. Grogan L, Tiernan M, Geogeghan N, Smyth B, Keenan E. Bloodborne virus infections among drug users in Ireland: a retrospective cross-sectional survey of screening, prevalence, incidence and hepatitis B immunisation uptake. Ir J Med Sci. 2005; 174:14-20.
5. Cullen W, Stanley J, D Langton, Y Kelly, G Bury. Management of hepatitis C among drug users attending general practice in Ireland: Baseline data from the Dublin Area Hepatitis C in General Practice initiative. Eur J of General Practice. 2007; 13:5-12.
6. Newell M.L, Pembrey L. Mother-to-child-transmission of hepatitis C virus infection. Drugs Today (Barc). 2002; 38:321-37.
7. Hupertz V.F., Wyllie R. Perinatal hepatitis C Infection. Paed IDJ. 2003; 22:369-72.
8. Healy CM, Cafferkey MT, Conroy A, Dooley S, Hall WW, Beckett M, Clarke TA, White MJ, Gorman WA, Butler KM. Outcome of infants born to hepatitis C infected women. Ir J Med Sci. 2001; 170:103-106
9. Ward C, Tudor-Willimas G, Cotzias T, Hargreaves S, Regan L, Foster G R. Prevalence of Hepatitis C among pregnant women attending an inner London obstetric department: uptake and acceptability of named antenatal testing. Gut. 2000; 47:277-280.
10. Kumar A, Aparna Sharma K Gupta RK, Kar P, Chakravarti A. Prevalence & risk factors for hepatitis C virus among pregnant women. Indian J Med Res. 2007; 211-215
11. Okamoto M, Nagata I, Murakami J, Kaji S, Iitsuka T, Hoshika T, Matsuda R, Tazawa Y, Shiraki K, Hino S. Prospective re-evaluation of risk factors in mother-to-child transmission of hepatitis C virus: high virus load, vaginal delivery, and negative anti-NS4 antibody. J Infect Dis. 2000; 182:1511-4
12. Tajiri H, Miyoshi Y, Funada S, Etani Y, Abe J, Onodera T, Goto M, Funato M, Ida S, Noda C, Nakayama M, Okada S. Prospective study of mother to infant transmission of hepatitis C virus. Pediatric Infect Dis. 2001; 20:10-14
13. Naomov NV. Hepatitis C virus infection in Eastern Europe. J Hepatol. 1999; 31(Suppl. 1):84-87.
14. Buligescu L, Mihaila M, Topala D. Significance of epidemiological ways of transmission of HCV infection. J Hepatol. 1992; 15:114-17.
15. Neumayr G, Propst A, Schwaighofer H, Judmaier G, Vogel W. Lack of evidence for the heterosexual transmission of hepatitis C. Q J Med. 1999; 92:505-508
16. Pembrey L, Newell M.L, Tovo1 P.A. Antenatal hepatitis C virus screening and management of infected women and their children: policies in Europe. European J of Pediatrics. 1999; 58:842-846
17. Esteban JI, Sauleda S, Quer J. The changing epidemiology of hepatitis C virus infection in Europe. J Hepatol. 2008; 48:148-62.
18. Anouk T. Urbanus, Thijs J.W. van de Laar, Anneke van den Hoek, Freke R. Zuure, Adrianus G.C.L. Speksnijder, Gijs G.G. Baaten, Titia Heijman, Henrike J. Vriend, Eline L.M. Op de Coul, Roel A. Coutinho, Maria Prins. Hepatitis C in general population of various ethnic origins living in the Netherlands: Should non-Western migrants be screened? Journal of Hepatology. 2011; 55:1207-1214
19. F Martyn, O Phelan, M O’Connell. Hepatitis C: Is There a Case of Universal Screening in Pregnancy? Ir Med J. 2011; 104:144-6.
20. Pembrey L, Newell M-L and Pecjham C. Is there a case for hepatitis C infection screening in the antenatal period? Journal of Medical Screening. 2003; 10:161-8
21. K.L.B. Reddick, R. Jhaveri, M. Gandhi, A.H. James, G.K. Swamy. Pregnancy outcomes associated with viral hepatitis. Journal of Viral Hepatitis. 2011; 18:e394-e398.
22. Pergam SA, Wang CC, Gardella CM, Sandison TG, Phipps WT, Hawes SE. Pregnancy complications associated with hepatitis C: data from a 2003– 2005 Washington state birth cohort. Am J Obstet Gynecol. 2008; 199:e1–e9.
23. Jabeen T,Cannon B,Hogan J,Crowley M,Devereux C,Fanning L,Kenny-Walsh E,Shanahan F,Whelton MJ. Pregnancy and pregnancy outcome in hepatitis C type 1b. Q J Med. 2000; 93:597–601.
24. Lin HH, Kao JH. Hepatitis C virus load during pregnancy and puerperium. BJOG. 2000; 107:1503–1506.
25. Irshad M, Khushboo I, Singh S, Singh S. Hepatitis C virus (HCV): a review of immunological aspects. Int Rev Immunol. 2008; 27:497–517.
|
|
|
|
Author's Correspondence
|
|
No Author Comments
|
|
|
Acknowledgement
|
|
No Acknowledgement
|
|
|
Other References
|
|
No Other References
|
|
|
|
|