|
|
|
|
|
|
|
|
AA Garvey,CP Hawkes,CA Ryan,M Kelly
|
|
|
|
Ir Med J. 2013 May;106(5):139-41
AA Garvey1, CP Hawkes1, CA Ryan1, M Kelly2
1Cork University Hospital, Wilton, Cork
2School of Medicine UCC, College Road, Cork
Abstract
Over-The-Counter Analgesics (OTCA) account for over a fifth of Irish pharmacy sales. Little is known about patterns of use, specifically in children. This study investigated parents’ use of OTCAs in children. A questionnaire exploring use of OTCAs and knowledge of side-effects was distributed to guardians of children attending three GP surgeries in South of Ireland from June-September 2010. The questionnaire was completed by 183 parents (response rate 95%). Many respondents (n=121, 66.1%) were using analgesics when not required or using an inappropriate analgesic for a child’s symptom. Private patients demonstrated better use (n=31, 40%) than those with Medical Cards (n=18, 22.5%) (p=0.016). Identification of potential side-effects was poor, with drowsiness (n=88, 49%), rash (n=39, 22%) and nausea (n=32, 18%) listed as potential side-effects. Inappropriate use of OTCAs is prevalent in Irish children. Parents need more information and guidance on their use.
|
|
Introduction
Over The Counter analgesics (OTCA) are among the most widely sold products in pharmacies, accounting for over a fifth of pharmacy sales in Ireland1. Pain, pyrexia and inflammatory musculoskeletal/joint conditions are the indications for OTCAs. Oral administration is the most common route of administration, but suppositories are preferred in vomiting children. They can be purchased directly from a pharmacy assistant/sales assistant without formal consultation. In an American study, over half of children under 3 years used OTCAs in the month of the study period2 and other studies show that parents/guardians often choose to medicate their children for minor illnesses without first seeking professional advice3,4. Parents are unsure of the correct dosing and possible side-effects of these medications5, and over half of all febrile children attending an Emergency Department in a US study, had previously received inaccurate doses of paracetamol or ibuprofen6. The 2010 Annual Report of the National Poisons Information Centre of Ireland, showed that of the 9330 enquiries regarding human poisoning, half concerned children less than 10 years of age7. Paracetamol was the most common drug reported followed by ibuprofen. While most paracetamol toxicity occurs as a result of a single overdose, toxicity can also occur as a result of numerous or repeated supra-therapeutic doses given at indicated intervals. Toxicity can be difficult to diagnose as early symptoms may mimic the underlying illness i.e. nausea, vomiting, anorexia and diaphoresis.
OTCA use, even when indicated and used appropriately, may have long-term health implications. There may be a relationship between paracetamol use in infancy and the development of atopic conditions8,9. Prophylactic use of paracetamol at the time of immunization has been shown to significantly reduce the primary antibody response to all serotypes of PCV 10 and to haemophilus influenza type b, diphtheria, tetanus and pertactin antigens10. For this reason, the Health Service Executive (HSE) of Ireland currently only recommends the use of paracetamol/ibuprofen for pyrexia >39.5°C or if a child has a large local reaction post-vaccination11. Several studies have described a relationship between OTCA use in children and socio-economic class, education and race2,12. Caucasian parents, parents with a higher level of education, and those with a higher income are more likely to use OTCAs. This increased use is also more prevalent among children whose parents do not have health insurance2. In addition, OTCA use for minor illnesses or for behavioural changes is more common in children whose parents work full-time12.
While children in Ireland commonly use OTCAs, little is known of their patterns of use; this study investigated parental use of OTCAs in children in Ireland.
Methods
A cross-sectional survey of parents and guardians with at least one child was undertaken during a 4-month period (June to September 2010) across three family physician surgeries in the South of Ireland. Reception staff at each of the sites invited all parents/guardians attending the medical centres with their children for appointments to complete a questionnaire. This was returned to the secretary in an anonymous, sealed envelope. A questionnaire was designed based on that of Allotey13. It was piloted and adjusted accordingly. Demographic data collected included age and marital status of parents/guardians, number of children under their care, health insurance status and age on leaving education. Questions regarding preferred types of OTCAs, knowledge of potential side effects and use in relation to immunisation were included. Use of OTCAs was assessed using 10 vignettes. Vignettes were constructed by a family doctor and paediatrician to reflect commonly encountered clinical scenarios. Participants indicated which analgesic, if any they would use in a particular situation. Participants were given the option of leaving a comment.
Results were dichotomized into appropriate or inappropriate use. Inappropriate use was defined as use of an analgesic that potentially did not optimise symptom control (e.g. oral medicines if a child is vomiting, and vice versa using suppositories if a child has diarrhoea) or use of analgesics not indicated (e.g. if a child is misbehaving, to induce sleep or to calm a child on long car journeys). Data was analysed with SPSS using a combination of both descriptive and inferential statistics. Chi squares were used to predict statistical relationships in which p-values <0.05 were taken to indicate a significant relationship. The Clinical Research Ethics Committee of the Cork Teaching Hospitals granted ethical approval.
Results
183 parents or guardians participated in this study; their mean age was 34 years (range 18-60 years). 9 parents declined to participate, citing time pressure. No questionnaire had more than 2 unanswered questions and all were included in subsequent analysis. All questions were answered in over 95% of questionnaires except educational status (74%) and insurance status (90%). Parents with and without health insurance, and those achieving each educational milestone were equally represented. Almost two thirds (62.3%) had more than one child and over 83% of participants indicated that their child/children attended a “Child minder”, Montessori, Pre-school or Primary school. An overview of results is given in
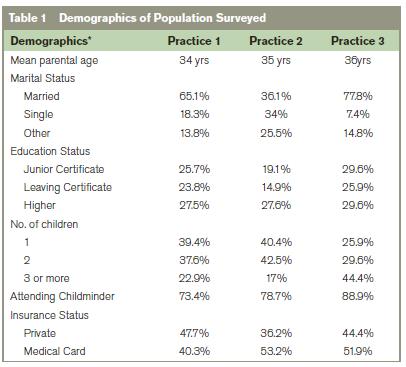
*Results were calculated as percentage of total population surveyed in cases of partial completion.
Measurement of temperature
Eighty-six percent of participants reported using a thermometer to measure temperature prior to administering OTCAs. Many used other methods such as feeling a child’s forehead (44.8%), instinct (22.9%) or the child’s behaviour (20.7%) to determine whether or not the child was pyrexial.
Source of medicines information
24% consulted a pharmacist, 19% a doctor, 7% a nurse, 86% always consulted the label prior to giving OTCAs.
Preparations Used
Oral formulations were most frequently used. Suppositories were used by 20.4% of parents/guardians. Of those not using suppositories, over half stated that they have never had reason to use them and would only use suppositories as a last resort as they felt they were “stronger” than the oral alternatives: 15.7% didn’t like administrating them and 2.2% of respondents had “never heard of them”.
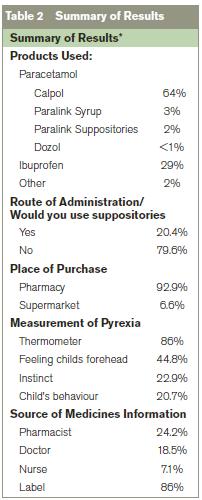
*Results were calculated as percentage of total population surveyed in cases of partial completion.
Clinical vignettes
Two thirds of respondents were using analgesics inappropriately, as determined by the clinical scenarios. 54% indicated that they would use the incorrect analgesic for a particular symptom and a third of respondents use OTC analgesics when there are no indications for use. Patients with private health insurance were more likely to have appropriate patterns of use (40%) compared to Medical Card/Doctor-Only Card holders (22.5%) (p=0.016).
Side-effects
Most parents (92.7%) purported that their child had never experienced side effects from OTCAs. However identification of potential side effects was poor, with drowsiness (49%), rash (22%) and nausea (18%) listed as the most commonly observed side effects. Approximately one in ten parents incorrectly indicated symptoms such as high temperature, headache and irritability as possible side effects. Almost 20% of parents/guardians said they would routinely give OTCA’s to their children prior to vaccinations.
Discussion
This study describes patterns of use of OTCAs in a paediatric population in Ireland. Pharmacies are the most common place of purchase. Parental misconceptions regarding correct route of administration, appropriate use, knowledge of side effects and use around time of vaccination, were observed. Previous studies have shown that many parents use OTCAs for unlicensed indications13. This was shown in our study, where one in three parents/guardians used OTCAs in situations in which they are not clinically indicated. In these instances, the OTCAs were used predominately for parental misconceived side effects, in particular sedation. Irish parents without private health insurance were more likely to incorrectly use OTCAs. This contrasts with an American study by Kogan et al2, which reported a higher level of incorrect use of OTCAs among those with private health insurance. Despite the fact that over 90% of OTCAs are purchased from pharmacies, only a quarter of parents/guardians regularly consulted a pharmacist prior to administration of OTCA. In addition, 14% do not routinely consult the label of the product prior to administration. Restricting OTCA availability to pharmacies, accompanied by a discussion with a pharmacist prior to sale, similar to measures implemented by the Pharmaceutical Society of Ireland (PSI) to increase awareness and reduce consumption of codeine containing products, could potentially result in improved use of OTCA amongst the Irish population.
In view of recent data assessing analgesic use and impaired immunological response to immunizations, it is reassuring that the majority of parents adhere to evidence-based guidelines and do not use OTCAs around the time of vaccination. Nevertheless, it remains common, with almost one fifth of those surveyed routinely administering OTCA prior to immunizations. This study consisted of almost 200 participants recruited from both urban and rural family practice surgeries, consistent with the current structure of primary care models in Ireland. As with all questionnaires, this study may have experienced recall bias in relation to OTCA use as it relied on a self-report method of data collection. In addition, the questionnaire was largely quantitative, such that explanations and causation factors for some of the research findings may not be generalisable.
In conclusion, incorrect OTCA use is prevalent in the Irish paediatric population. We recommend that all sellers of OTCAs should be aware of their responsibilities and use the point of sale to provide and reinforce correct information to parents. Finally, all healthcare providers should enquire about OTCA use at routine healthcare visits allowing for opportunistic parental education.
Correspondence: M Kelly
G329 Undergraduate Family Medicine, Health Sciences Centre, University of Calgary, 3330 Hospital Drive, Calgary NW, Alberta T2N 2N1, Canada
Email: [email protected]
References
1. Fisher CM, Henman MC, Corrigan OI. A study of community pharmacy practice, 2: Prescription Dispensing. Journal of Social and Administrative Pharmacy. 1991;8:65-8.
2. Kogan MD, Pappas G, Yu SM, Kotelchuck M. Over-the-counter medication use among US preschool-age children. JAMA. 1994 Oct 5;272:1025-30.
3. Cantrill JA, Johannesson B, Nicolson M, Noyce PR. Management of minor ailments in primary schoolchildren in rural and urban areas. Child Care Health Dev. 1996 May;22:167-74.
4. Ames JT, Hayden GF, Campbell RE, Lohr JA. Parents' conception of their use of over-the-counter medicines. Clin Pediatr (Phila). 1982 May;21:298-301.
5. Simon HK, Weinkle DA. Over-the-counter medications. Do parents give what they intend to give? Arch Pediatr Adolesc Med. 1997 Jul;151:654-6.
6. Li SF, Lacher B, Crain EF. Acetaminophen and ibuprofen dosing by parents. Pediatr Emerg Care. 2000 Dec;16:394-7.
7. Poisons Information Centre of Ireland. Annual Report. Available from http://www.poisons.ie2010.
8. Farquhar H, Stewart A, Mitchell E, Crane J, Eyers S, Weatherall M, Beasley R. The role of paracetamol in the pathogenesis of asthma. Clin Exp Allergy. 2010 Jan;40:32-41.
9. Lowe AJ, Carlin JB, Bennett CM, Hosking CS, Allen KJ, Robertson CF, Axelrad C, Abramson MJ, Hill DJ, Dharmage SC. Paracetamol use in early life and asthma: prospective birth cohort study. BMJ. 2010;341:c4616.
10. Prymula R, Siegrist CA, Chlibek R, Zemlickova H, Vackova M, Smetana J, Lommel P, Kaliskova E, Borys D, Schuerman L. Effect of prophylactic paracetamol administration at time of vaccination on febrile reactions and antibody responses in children: two open-label, randomised controlled trials. Lancet. 2009 Oct 17;374:1339-50.
11. Health Service Executive [homepage on the internet]. Ireland. http://www.immunisation.ie/en/ChildhoodImmunisation/YourQuestionsAnswered/PostVaccination/ accessed 8/12/2011.
12. Slack-Smith LM, Read AW, Stanley FJ. The use of medication in children attending childcare in Western Australia. J Paediatr Child Health. 1998 Apr;34:183-7.
13. Allotey P, Reidpath DD, Elisha D. "Social medication" and the control of children: a qualitative study of over-the-counter medication among Australian children. Pediatrics. 2004 Sep;114:e378-83.
|
|
|
|
Author's Correspondence
|
|
No Author Comments
|
|
|
Acknowledgement
|
|
No Acknowledgement
|
|
|
Other References
|
|
No Other References
|
|
|
|
|